pH: Definition and Measurement
Are you tired of writing out really tiny numbers in scientific notation to express the concentrations of H3O+ and OH-? Is all of the multiplying and dividing of those tiny numbers giving your wrist spasms? You're in luck. Brilliant French-speaking chemists are now offering the pH scale for all your acid and base chemistry needs.
The pH scale is used to express the concentration of H3O+ in acidic or basic solutions. The scale is logarithmic so the "p" in pH denotes that it's the power of 10 or the power of hydrogen as the French might say. The following definition should be etched into your brain:
pH = -log[H3O+] or [H3O+] = 10-pH
The equation above tells us the a neutral solution (at 25 °C), which has a H3O+ concentration of 1 × 10-7 M, will have a pH of 7 [-log(1 × 10-7)]. An acidic solution with 1 × 10-4 M H3O+ will have a pH of 4. A basic solution with 1 × 10-10 M H3O+ will have a pH of 10. If you find yourself without a calculator and need to convert between [H3O+] and pH it helps to pay attention to exponents. Notice that the exponents in the examples above are the pH values.
Another useful trick to use when dealing with pH is to think in log units. This has nothing to do with lumberjacks. We're talking about thinking in terms of factors of 10. For example, if new have one solution with a pH of 5 and another solution with a pH of 6, their concentration of H3O+ differ by 10-fold. In other words, the pH 5 solution has 10 times more H3O+ than the pH 6 solution.
To really appreciate the logarithmic nature of the pH scale, let's think about a real world example.
Say we want to change the pH of a large public swimming pool. To change the pH by 1 pH unit, for example from 8 to 7, we would need only about a 1 L bottle of concentrated strong acid to get the job done. However, if we needed to rebalance the pH from 11 to 7 we would need 1000 times more acid to achieve just the 4 pH unit change. That's 1000 L of strong acid that would need to be added. This would require renting a U-Haul and wearing really cool Hazmat suits.

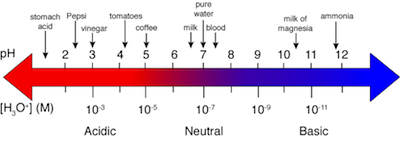
Here's another thing to keep in mind about the pH scale. Because it's a negative log of the H3O+ concentration, the pH decreases as the concentration of H3O+ increases. Therefore, solutions with low pH values have high concentrations of acid. Inversely, solutions with high pH values have low concentrations of acid. The figure below shows the pH values of some familiar substances illustrating the negative log relationship between [H3O+] and pH.
The pOH Scale
While the pH scale is the conventional scale that's usually used in chemistry, it's also possible to define the pOH scale in a similar way. The pOH scale indicates the concentration of OH- in negative log units:
pOH = -log[OH-] or [OH-] = 10-pOH
A solution with a high pOH has a low concentration of OH- and a solution with a low pOH has a high concentration of OH-. A helpful relationship is that the pH and pOH of a particular solution will always add up to 14 (at 25 °C where Kw = 1 × 10-14). So if you know the pH of a solution, just subtract that number from 14 to find the pOH. If you know the pOH of a solution, just subtract it from 14 to find the pH.
The deal is this: we don't count H3O+ molecules ourselves. Instead, we use special chemicals called acid-base indicators to do the dirty work. These special chemicals change colors based on the concentration of H3O+ in the solution with them. Here's a generic chemical equation illustrating this:
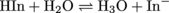
The indictor (HIn) is a weak acid so there is an equilibrium between its form with a proton (called the protonated form) and its form without the proton (called the deprotonated form). The protonated form (the acid) emits one color of light, for example yellow. The deprotonated form (the conjugate base) emits a different color of light, for example blue. In the example above, if there is a lot of acid in the solution, the reactant side will be favored and the solution would be yellow. On the other had, if there is not much acid, the product side will be favored and the solution will be blue.
Typical indicators will have a visible color change over about 1-2 units. For example, Bromothymol blue changes from yellow at pH values below 6.1 to blue at pH values above 8.1. At pH values in between 6.1 and 8.1, the color will appear as a mixture of yellow and blue, which would be green just like a Glad-lock zipper bag.
Not to get all mathematical on you, but it might help to think about this with some numbers. For the indicator bromothymol blue, at pH 7.1 there are equal parts of protonated bromothymol blue (which is yellow) and deprotonated bromothymol blue (which is blue) so the observed color is green.
However, if we go one pH unit lower to 6.1, the concentrations of molecules changes by a factor of 10. For every nine molecules of protonated bromothymol blue there will only be one molecule of deprotonated bromothymol blue. To the human eye the color will look like pure yellow since the blue signal of the one deprotonated bromothymol blue molecule will be masked by the yellow signal from the more abundant protonated bromothymol blue molecules.
If we were to go one pH unit higher from pH 7.1 to 8.1, the equilibrium moves in the opposite direction so that the blue molecules overwhelm the signal from the yellow molecules and the solution appears pure blue above pH 8.1.
Acid-base indicators such as the bromothymol blue example are generally good at measuring the pH of a solution to within ±1 pH unit. By mixing a cocktail of indictors, we can determine the pH of a solution over a wide pH range. These cocktails are called universal indicators and are typically infused onto pH strips that are dipped into solutions and change colors to reflect the pH.

Example of universal pH indictor on a paper roll. (Image from here.)
When chemists need a more precise measurement of pH, they use pH meters to measure the concentration of H3O+. The inner workings of pH meters are complex and rely on electrochemical principles to measure proton potential gradients. (Yeah, we don't want to get into it, either.) Just know that pH meters are really handy instruments and can report the pH of a solution to ±0.01 pH unit.
The pH scale is used to express the concentration of H3O+ in acidic or basic solutions. The scale is logarithmic so the "p" in pH denotes that it's the power of 10 or the power of hydrogen as the French might say. The following definition should be etched into your brain:
pH = -log[H3O+] or [H3O+] = 10-pH
The equation above tells us the a neutral solution (at 25 °C), which has a H3O+ concentration of 1 × 10-7 M, will have a pH of 7 [-log(1 × 10-7)]. An acidic solution with 1 × 10-4 M H3O+ will have a pH of 4. A basic solution with 1 × 10-10 M H3O+ will have a pH of 10. If you find yourself without a calculator and need to convert between [H3O+] and pH it helps to pay attention to exponents. Notice that the exponents in the examples above are the pH values.
Another useful trick to use when dealing with pH is to think in log units. This has nothing to do with lumberjacks. We're talking about thinking in terms of factors of 10. For example, if new have one solution with a pH of 5 and another solution with a pH of 6, their concentration of H3O+ differ by 10-fold. In other words, the pH 5 solution has 10 times more H3O+ than the pH 6 solution.
To really appreciate the logarithmic nature of the pH scale, let's think about a real world example.
Say we want to change the pH of a large public swimming pool. To change the pH by 1 pH unit, for example from 8 to 7, we would need only about a 1 L bottle of concentrated strong acid to get the job done. However, if we needed to rebalance the pH from 11 to 7 we would need 1000 times more acid to achieve just the 4 pH unit change. That's 1000 L of strong acid that would need to be added. This would require renting a U-Haul and wearing really cool Hazmat suits.

Here's another thing to keep in mind about the pH scale. Because it's a negative log of the H3O+ concentration, the pH decreases as the concentration of H3O+ increases. Therefore, solutions with low pH values have high concentrations of acid. Inversely, solutions with high pH values have low concentrations of acid. The figure below shows the pH values of some familiar substances illustrating the negative log relationship between [H3O+] and pH.

The pOH Scale
While the pH scale is the conventional scale that's usually used in chemistry, it's also possible to define the pOH scale in a similar way. The pOH scale indicates the concentration of OH- in negative log units:
pOH = -log[OH-] or [OH-] = 10-pOH
A solution with a high pOH has a low concentration of OH- and a solution with a low pOH has a high concentration of OH-. A helpful relationship is that the pH and pOH of a particular solution will always add up to 14 (at 25 °C where Kw = 1 × 10-14). So if you know the pH of a solution, just subtract that number from 14 to find the pOH. If you know the pOH of a solution, just subtract it from 14 to find the pH.
Measuring Sticks for pH
We saw above that pH is just an expression of the concentration of H3O+ in a solution. How we can we find out the pH of a solution? After all, we can't count all the H3O+ molecules in a given solution on our fingers and toes. There are too many H3O+ molecules (in 1 mL of water there are about 6 × 1013 molecules of H3O+) and they are too tiny to see. What's the deal?The deal is this: we don't count H3O+ molecules ourselves. Instead, we use special chemicals called acid-base indicators to do the dirty work. These special chemicals change colors based on the concentration of H3O+ in the solution with them. Here's a generic chemical equation illustrating this:
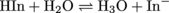
The indictor (HIn) is a weak acid so there is an equilibrium between its form with a proton (called the protonated form) and its form without the proton (called the deprotonated form). The protonated form (the acid) emits one color of light, for example yellow. The deprotonated form (the conjugate base) emits a different color of light, for example blue. In the example above, if there is a lot of acid in the solution, the reactant side will be favored and the solution would be yellow. On the other had, if there is not much acid, the product side will be favored and the solution will be blue.
Typical indicators will have a visible color change over about 1-2 units. For example, Bromothymol blue changes from yellow at pH values below 6.1 to blue at pH values above 8.1. At pH values in between 6.1 and 8.1, the color will appear as a mixture of yellow and blue, which would be green just like a Glad-lock zipper bag.
Not to get all mathematical on you, but it might help to think about this with some numbers. For the indicator bromothymol blue, at pH 7.1 there are equal parts of protonated bromothymol blue (which is yellow) and deprotonated bromothymol blue (which is blue) so the observed color is green.
However, if we go one pH unit lower to 6.1, the concentrations of molecules changes by a factor of 10. For every nine molecules of protonated bromothymol blue there will only be one molecule of deprotonated bromothymol blue. To the human eye the color will look like pure yellow since the blue signal of the one deprotonated bromothymol blue molecule will be masked by the yellow signal from the more abundant protonated bromothymol blue molecules.
If we were to go one pH unit higher from pH 7.1 to 8.1, the equilibrium moves in the opposite direction so that the blue molecules overwhelm the signal from the yellow molecules and the solution appears pure blue above pH 8.1.
Acid-base indicators such as the bromothymol blue example are generally good at measuring the pH of a solution to within ±1 pH unit. By mixing a cocktail of indictors, we can determine the pH of a solution over a wide pH range. These cocktails are called universal indicators and are typically infused onto pH strips that are dipped into solutions and change colors to reflect the pH.

Example of universal pH indictor on a paper roll. (Image from here.)
When chemists need a more precise measurement of pH, they use pH meters to measure the concentration of H3O+. The inner workings of pH meters are complex and rely on electrochemical principles to measure proton potential gradients. (Yeah, we don't want to get into it, either.) Just know that pH meters are really handy instruments and can report the pH of a solution to ±0.01 pH unit.