Mitochondrial Membranes
Mitochondria, like nuclei, have two phospholipid bilayers. The bilayer closest to the cytoplasm, or the outer mitochondrial membrane (OMM, not Om Nom Nom), has integral proteins called porins that allow small molecules to pass freely into the mitochondria.
These molecules do not get very far, though, because they soon encounter the second bilayer, or inner mitochondrial membrane (IMM). This membrane is folded and twisted throughout the mitochondrion into structures called cristae, much like all 25 feet of your small intestine is twisted and folded throughout your abdomen. The space inside the IMM is called the matrix, while the space between the two mitochondrial membranes is cleverly named the intermembrane space. Since the OMM has many porins, the intermembrane space closely resembles the physical and chemical properties of the cytoplasm.
For those that missed it the first time or just plain forgot, here is a mitochondrion:

As you may, and should, recall from the section on mitochondria, these organelles are chiefly responsible for converting the chemical energy in macromolecules, like glucose, into molecules of ATP, adenosine triphosphate, that can be used by the cell for energy. The ability of a mitochondrion to convert glucose and ADP, adenosine diphosphate, into ATP is intricately connected to the structure of the mitochondrial membranes.
In a process called glycolysis, which occurs in the cytoplasm just outside the mitochondrion, electrons are stripped from glucose and passed through the outer mitochondrial membrane into the intermembrane space. Here, the electrons are passed to a series of special proteins embedded in the IMM. As the electrons move from one membrane protein to the next, energy is released and protons (hydrogen ions, or H+) in the matrix are pumped across the IMM and into the intermembrane space. Fairly quickly, a large number of protons accumulate in the intermembrane space and, like water behind a dam, exert great pressure on the IMM.
Luckily for them, and life as we know it, there is a special protein complex embedded in the IMM that allows protons to flow back into the matrix. The special part about this channel protein complex is that it is capable of harnessing the enormous energy produced by the rush of protons. Exactly like a turbine in a dam, ATP synthase—as this protein complex is named, and yes, it is an enzyme (–ase)—has a rotor that spins when protons push past.
Can't visualize what we mean? Here's a picture:
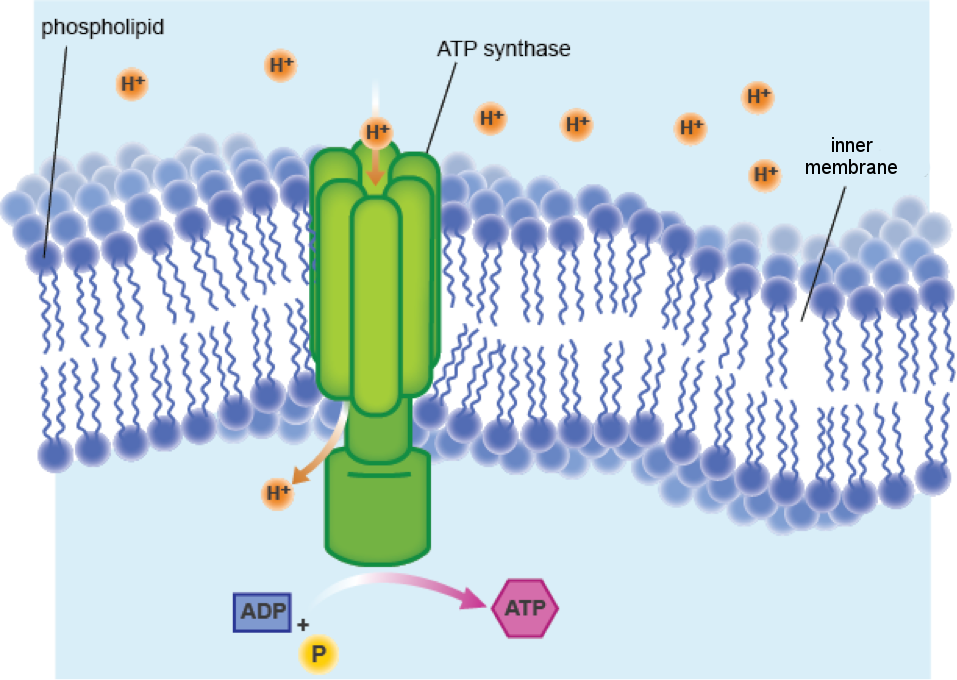
The energy generated by the turning of the rotor is converted into ATP, just like the energy made by turning a water turbine in a dam is converted into electricity. In this way, one molecule of glucose can be converted into about 38 molecules of ATP. A pretty good investment, if you ask us. This awesome process is called cellular respiration, and it is all made possible by the mitochondrial membranes!
Brain Snack
The mitochondrial membrane is the site of ATP synthesis, and the ATP made is inside the mitochondrion. How do you think it gets to the cytoplasm so that it can be used? Naturally, it gets where it needs to go by using those transmembrane channels we discussed earlier.
These molecules do not get very far, though, because they soon encounter the second bilayer, or inner mitochondrial membrane (IMM). This membrane is folded and twisted throughout the mitochondrion into structures called cristae, much like all 25 feet of your small intestine is twisted and folded throughout your abdomen. The space inside the IMM is called the matrix, while the space between the two mitochondrial membranes is cleverly named the intermembrane space. Since the OMM has many porins, the intermembrane space closely resembles the physical and chemical properties of the cytoplasm.
For those that missed it the first time or just plain forgot, here is a mitochondrion:

As you may, and should, recall from the section on mitochondria, these organelles are chiefly responsible for converting the chemical energy in macromolecules, like glucose, into molecules of ATP, adenosine triphosphate, that can be used by the cell for energy. The ability of a mitochondrion to convert glucose and ADP, adenosine diphosphate, into ATP is intricately connected to the structure of the mitochondrial membranes.
In a process called glycolysis, which occurs in the cytoplasm just outside the mitochondrion, electrons are stripped from glucose and passed through the outer mitochondrial membrane into the intermembrane space. Here, the electrons are passed to a series of special proteins embedded in the IMM. As the electrons move from one membrane protein to the next, energy is released and protons (hydrogen ions, or H+) in the matrix are pumped across the IMM and into the intermembrane space. Fairly quickly, a large number of protons accumulate in the intermembrane space and, like water behind a dam, exert great pressure on the IMM.
Luckily for them, and life as we know it, there is a special protein complex embedded in the IMM that allows protons to flow back into the matrix. The special part about this channel protein complex is that it is capable of harnessing the enormous energy produced by the rush of protons. Exactly like a turbine in a dam, ATP synthase—as this protein complex is named, and yes, it is an enzyme (–ase)—has a rotor that spins when protons push past.
Can't visualize what we mean? Here's a picture:

The energy generated by the turning of the rotor is converted into ATP, just like the energy made by turning a water turbine in a dam is converted into electricity. In this way, one molecule of glucose can be converted into about 38 molecules of ATP. A pretty good investment, if you ask us. This awesome process is called cellular respiration, and it is all made possible by the mitochondrial membranes!
Brain Snack
The mitochondrial membrane is the site of ATP synthesis, and the ATP made is inside the mitochondrion. How do you think it gets to the cytoplasm so that it can be used? Naturally, it gets where it needs to go by using those transmembrane channels we discussed earlier.