The Theme of Structure and Function in Biomolecules and the Chemistry of Life
We have talked a lot about the structures and functions of biological molecules, but 'til now, we haven’t spent much time thinking about how structure and function relate to each other. Don't fret; biological molecules provide some great examples of this theme.
Take the phospholipid bilayer. Recall that phospholipids are one kind of lipid; they have a glycerol backbone with two fatty acid "tails" and a phosphate "head." How does the structure of a phospholipid allow it to carry out its function?
1. The fact that the tails are hydrophobic means that they do not interact with water. When a bunch of phospholipids are floating around in water, they try to arrange themselves in a bilayer that shields the hydrophobic parts from water-based, or aqueous, surroundings.
2. The heads are hydrophilic and can then interact with water and other polar or charged substances on either side of the bilayer. The bilayer acts as a barrier that allows cells to maintain internal conditions that are different from external conditions, which is monumentally important for cells to operate properly. Everything from nerve impulse conduction to muscle firing to cellular metabolism depends on the cell's ability to maintain different conditions on opposite sides of the bilayer.
3. Phospholipids demonstrate the intersection of structure and function in another way, too. We already know that fatty acids can be saturated or unsaturated and that unsaturated fatty acids have bends in their chains. Those bends prevent fatty acids from packing closely together, which causes cell membranes (membrane = phospholipid bilayer + other stuff) that contain lots of unsaturated fatty acids to be more "fluid." Fluid describes fatty acids that cannot pack in as tightly, and as a result, they move more freely over the surface of the cell.
It might be weird to think about cell membranes as fluids, but actually, this property is really important for proper membrane functioning. Enzymes need to move around in order to work, and if a membrane is not fluid enough, it might become impermeable (walled off) to certain substances that normally pass through the bilayer easily. In sum, the fact that phospholipids structurally have polar and nonpolar parts, and the fact that fatty acids can structurally be saturated or unsaturated, allow phospholipid bilayers to properly function in regulating a cell’s contents.
Proteins provide another great example of the intersection between structure and function. As we already know, proteins have four levels of structure: primary, or the sequence of amino acids; secondary, or the coils and folds from bonds between backbone elements; tertiary, or the coils and folds from bonds between R groups; and quaternary, or the conglomeration of more than one folded subunit. A protein is not a protein without its 3D structure, and regardless of what function it has, a protein is useless if its structure is somehow incorrect. Scientists in the field actually refer to proteins as having structure-function relationships. You don't get more thematic than that.
Enzymes, for example, function by binding to a particular substrate, or substance. If the enzyme is misshapen, the substrate will not be able to find its binding site on the enzyme, and that reaction will never be catalyzed, or kick-started. Cellular receptors are also good examples. Receptors function much in the same way as enzymes: they have a specific site where a signal molecule can bind, and once that signal is bound, it sets off a chain reaction that conveys information to other parts of a cell, or to other cells in the organism. If a receptor protein has the wrong shape, the signal molecule cannot bind, and the flow of information will cease. That spells trouble for the cell.
What causes a protein to have the wrong shape? There are a couple conditions that can cause this misshaping to happen. If a mutation (read: unexpected alteration) in the genetic code causes the wrong amino acids to be incorporated into the protein’s primary structure, later folding may be affected by that change. Sickle cell anemia is a blood disease caused by an incorrect amino acid in one of the subunits of hemoglobin.
Hemoglobin has two subunits, and together, the protein's combined function is to bind to oxygen and deliver it to cells all over your body. Hemoglobin is present in your red blood cells, or RBCs for short, that circulate through your blood vessels, delivering oxygen to all your organs and tissues. People with sickle cell anemia have a valine amino acid instead of a glutamic amino acid at a certain position along the polypeptide chain. This alteration causes improper folding of the hemoglobin subunit that leads to two problems:
- Reduced oxygen-carrying capacity
- Red blood cells that have the wrong shape
Yes, you read correctly. In this case, proteins of the wrong shape lead to whole cells that are the wrong shape! Those misshapen cells clog blood vessels and prevent proper circulation.
Even if the sequence of the amino acids in the polypeptide chain is correct, proteins can still fold incorrectly if they are surrounded by bad influences. When proteins are forming and taking on their 3D shapes, they can be strongly influenced by environmental factors. Yes, proteins experience peer pressure, too. If environmental conditions are poor, blossoming proteins may go down the wrong path and fold incorrectly, rendering themselves useless. Luckily, there are special proteins, called chaperonins, that help other proteins fold correctly. Chaperonins are shaped like little capsules, and they provide a safe environment for proteins to fold correctly, away from all the bad outside influences. Once the synthesized proteins are properly formed in terms of structure, they are released into the wider world of the body they live in and carry out their functions normally.
Here is how a chaperonin usually operates.
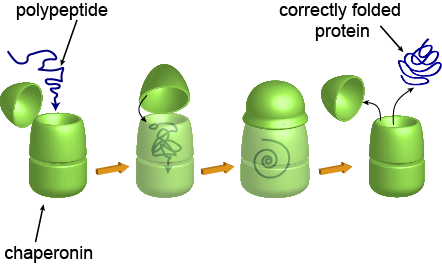
And here is what a chaperonin usually looks like.

As we have seen, structure and function relate to each other in different ways in lipids and proteins. Might there be other ways in which structure and function unite in carbohydrates? How about in nucleic acids? Keep thinking about structure and function as you learn more about DNA replication and protein synthesis.