Bonding: Covalent and Ionic Bonds
Get Ready for some Bonding Time
Ever feel overwhelmed with tests and homework? Do you wish you could balance all the things in your life a little better? We all need some stability in our lives. Atoms need stability, too. Atoms are constantly trying to complete their outer shell (find balance), and they will lose, gain, or even share electrons in order to achieve maximum stability.In order to complete their outer shell, some atoms give electrons, some atoms share electrons, and some atoms steal electrons. An atom's placement in the periodic table dictates what kind of personality that atom is likely to have (a giver, sharer, or a stealer).
Exactly what types of bonds are formed between atoms to create compounds?
Here are some general guidelines to apply:
Metals + Nonmetals → Ionic Bonds
Nonmetal + Nonmetal → Covalent Bonds
Metal + Metal → Metallic Bonds
Ionic Bonding
An ionic bond is held together by the electrostatic attraction between ions that are near one another. Electrostatic attraction is the attraction between atoms that have opposite charge and holds the atoms together in ionic bonds. Think of it as an atomic glue. In this type of bond, one atom gives up electrons and becomes a positively charged ion (cation). Another atom dons a ski mask and steals the electrons to become a negatively charged ion (anion). Okay, maybe steals is a bit harsh. The atom actually "accepts" or "takes" the electrons that the other atom is giving up. (We don't want to be accused of name-calling.)Some of our favorite foods have a bunch of ionic bonds sprinkled all over them. Is anyone up for some chips, fries, pretzels, or peanuts? Notice a pattern? #yumsalt
Salts, including table salt, are held together by ionic bonds. Table salt is made of sodium chloride, or NaCl.
Take a look at the periodic table. Notice that sodium is in the first column of the periodic table. That means it has only one valence electron in its outer shell. Chlorine, on the other hand, is in the seventh column. It has seven valence electrons. Cl only needs one more electron to complete its outer shell. Did you play matchmaker in your head? Sodium gladly gives up its extra valence electron to chlorine, which gobbles it up faster than Takeru Kobayashi eats hotdogs. The sodium atom becomes a cation and the chlorine atom because an anion, and the two atoms are held together by the positive and negative charges of the ions.

According to Coulomb's law, more highly charged ions form stronger bonds than less highly charged ions. Ionic bonds tend to be fairly strong and therefore have relatively high melting and boiling points. Ionic solids are poor conductors of electricity because each electron is localized around a particular atom. Their electrons aren't swimming in the electron pool. As a result, they don't move around the lattice. Ionic liquids do conduct electricity, however, because the ions are free to move around in the liquid phase.
Covalent Bonding
In the case of covalent bonds, electrons are neither lost nor gained. No ions are formed. Instead, the electrons are shared. This group of atoms must have watched Sesame Street as a kid. Covalent bonding is the sharing of one or more pairs of electrons between nonmetals.Covalent bonds in a molecule are often depicted as a dash. The dash represents the two electrons shared between the atoms. The atoms can have a:
| Bond | Sharing how many e-? | Example |
| Single bond | Sharing one pair of electrons | H–H |
| Double bond | Sharing two pairs of electrons | H2C=CH2 |
| Triple bond | Sharing three pairs of electrons | N≡N |
There are two types of covalent bonds. The first is called a non-polar covalent bond. This is where the atoms equally attract and share the electrons. An example of this is two hydrogen atoms (H—H) sharing a pair of electrons. Each hydrogen nucleus has one proton that equally "tugs" or attracts the bonding pair of electrons.
The second type of covalent bond is the polar covalent bond. This is where the two atoms in the covalent bond are not the same and the attraction to the electron pair is not equal. The atom with the greater attraction pulls the bonding electrons towards them. As a result, the atom takes on a partial negative charge. The other atom has less attraction for the bonding electrons, and it will take on a partial positive charge. An example of this is HCl. The chlorine atom has a much greater attraction for the bonding pair of electrons and will therefore take on a partial negative charge.

This type of bond is sometimes depicted as:
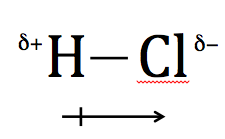
Instead of using a single line to indicate the covalent bond, an arrow pointing towards the atom that has the greater attraction for the electron pair is used.
The attractive force an atom has on a bonding pair of electrons is referred to as the atom's electronegativity. It's a measure of how badly the atom wants an electron. Atoms like chlorine and fluorine in that second to the last column on the right are desperate for electrons to complete their octet.
In fact, electronegativity values have been calculated for all the atoms (see table below) so we can see which ones are more desperate than others for that extra electron. A general rule, as we go from left to right on the periodic table the electronegativity increases, as seen with the H and Cl atoms above. The electronegativity values can also be used to predict whether two atoms will likely undergo ionic, polar covalent, or non-polar covalent bonds.
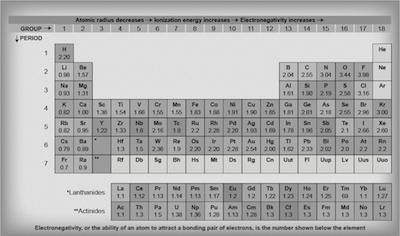
Refer to this video to get a better idea of how to tell if two atoms are likely to contain covalent versus ionic bonds as a result of their electronegativity.