ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Physics 2: 2.2 Changes and Conservation Laws 10 Views
Share It!
Description:
AP Physics 2: 2.2 Changes and Conservation Laws. Which of the following is not one of the possible energy levels for double-ionized lithium?
Transcript
- 00:04
And here's your shmoop du jour brought to you by repetition [Man with a paddle hitting a ball]
- 00:06
repetition repetition...All right some of the info in this drill was part of the
- 00:11
last drill but don't worry we're not going to repeat ourselves yeah we just [People watching a screen]
- 00:14
need to be aware that some of the info in this drill was part of the last drill
- 00:17
but we're not going to repeat ourself... we did deja vu there all
Full Transcript
- 00:21
right moving on okay, the ionization energy for double ionized lithium which
- 00:26
has the equal three protons is negative 13.6 electron volts which of the
- 00:32
following is not one of the possible energy levels for double ionized lithium
- 00:36
and here are the potential answers.... okay if you watch the last video the info on the [A couple watching protons spinning]
- 00:43
number of protons and ionization energy will sound familiar if you didn't watch
- 00:46
it well go check it out it's fantastic you'll laugh you'll cry and you'll just [People laughing]
- 00:50
learn a thing or two maybe and more importantly you'll have the math to
- 00:54
solve this question well in that last question we use Bohr's formula to find
- 00:58
the energy level of this atom the formula is e sub N equals Z squared over
- 01:02
N squared times the ground state energy level looks like that with Z at three [Bohr's equations on a board]
- 01:08
and a ground state energy level equaling the ionization energy negative thirteen
- 01:12
point six we know that the energy levels equal 9 over N squared times negative 13
- 01:17
point 6 well now we can just plug in numbers for n to see what works and what
- 01:21
doesn't if n equals 1 we find that the energy level equals negative 122.4
- 01:26
electron volts answer B) is a possible energy level so it's not the right
- 01:30
answer as we plug in more numbers will find that A) and C) are the n values of 2 [Answers A and C flashing]
- 01:35
and 5 respectively so those answers are out too but there's no whole number n
- 01:40
value that'd make D) a possible answer so that's the correct answer for this
- 01:44
question - N will always be a whole number after all there's no such thing
- 01:48
as energy level one and a half so it's not too hard to figure out the energy [Girl reading energy level book]
- 01:53
level as long as we know the atomic number and the ground state energy it
- 01:57
might take a little trial and error but we'll be able to do the math and get it [Boy stood by a slot machine]
- 02:00
right.. Sure, it might feel a little repetitive changing out the value for n
- 02:03
a few times but let's face it repetition is a fact of life in science and math so
- 02:08
as we were saying and here's your shmoop du jour brought to you by...[Man hitting ball with a paddle]
Up Next
Related Videos
AP Physics 2: 1.1 Properties of Objects and Systems. What is the magnitude and direction of the conventional current in this wire?
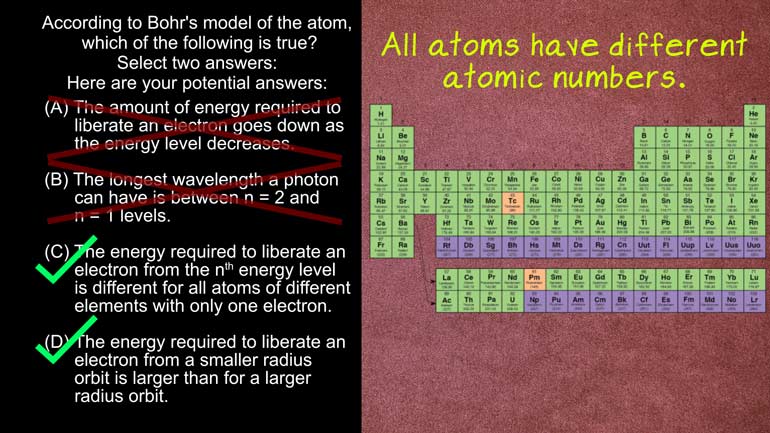
AP Physics 2: 1.5 Properties of Objects and Systems. According to the Bohr's model of the atom, which of the following are true?
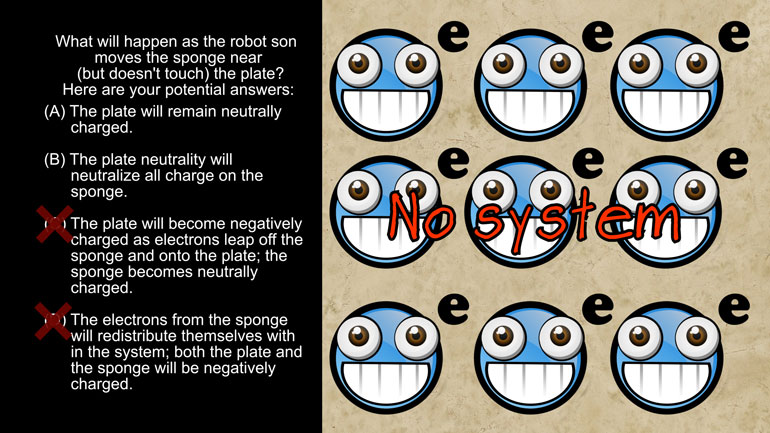
AP Physics 2: 2.2 Properties of Objects and Systems. What will happen as the robot son moves the sponge near (but doesn't touch) the plate?
AP Physics 2: 2.4 Properties of Objects and Systems. How could you show the carnival barker an emission spectrum?