ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Physics 2: 2.5 Probability and Systems 177 Views
Share It!
Description:
AP Physics 2: 2.5 Probability and Systems. In which of these cases does the entropy increase?
Transcript
- 00:00
Thank you We sneak and here's your shmoop douceur brought
- 00:05
to you by entropy with fancy word for chaos All
- 00:10
right consider the following situations A pile of paper gets
- 00:13
organized The lake freezes to gases are brought together and
- 00:16
allowed teo mix that a gas undergoes a cycle like
Full Transcript
- 00:22
the one described in this pv diagram here In which
- 00:25
of these cases does the intra pete increase select two
- 00:29
answers And here the choice What No complicated equations No
- 00:36
big number times ten to the million Physics is all
- 00:39
about real life and interviews all too real Well entropy
- 00:43
is a measure of order when the lack thereof the
- 00:46
more order to system is the less entropy it has
- 00:49
Conversely the less ordered a system is the more intricate
- 00:52
hat accent right now interview can increase but it can't
- 00:56
decrease entropy is part of the second law of thermodynamics
- 01:00
and simplify it Entropy means that work can be converted
- 01:04
perfectly into heat but he cannot be converted perfectly into
- 01:09
work Well the most important part of entropy for the
- 01:12
test is this entropy can stay the same if in
- 01:15
action or process is reversible if the process is not
- 01:19
reversible than entropy increases So let's look at our examples
- 01:23
First of all organizing a pile of papers results in
- 01:26
decreased interview wait didn't we just say entropy can't decreased
- 01:30
Yeah we did In this example the act of organizing
- 01:33
the papers creates heat energy that dissipates that's a loss
- 01:37
of energy in the process of work In the best
- 01:39
case scenario is that the total entropy remains the same
- 01:43
This case though we're going from a state of less
- 01:46
organization more organization the entropy shouldn't increase is not one
- 01:51
of the correct choice What about the late going from
- 01:54
liquid to solid As it freezes well again entropy isn't
- 01:58
decreasing After all solids are more organized than those wild
- 02:01
and crazy liquid it's watching around everywhere This is a
- 02:04
reversible processes we'll see When spring comes the baby is
- 02:08
also incorrect when two gases come together and mix though
- 02:11
that's not reversible You can't just go out and grab
- 02:16
all the molecules and put them one by one back
- 02:18
in there to original gases see definitely increases entropy Andy
- 02:24
shows a heat engine cycle that isn't reversible either really
- 02:27
the on ly reversible heat engine cycle is a car
- 02:30
not cycle and that's only theoretical and not possible in
- 02:34
real life so not reversible equals increase entropy He is
- 02:39
the other correct answer And remember next time your mom
- 02:42
tells you to clean up the mess in your room
- 02:44
just tell her that the universe always moves towards disorder
- 02:47
and your action and clean can only stall the collapse
- 02:49
not prevented so like why bother And if that works 00:02:53.233 --> [endTime] please let us know
Up Next
Related Videos
AP Physics 2: 1.1 Properties of Objects and Systems. What is the magnitude and direction of the conventional current in this wire?
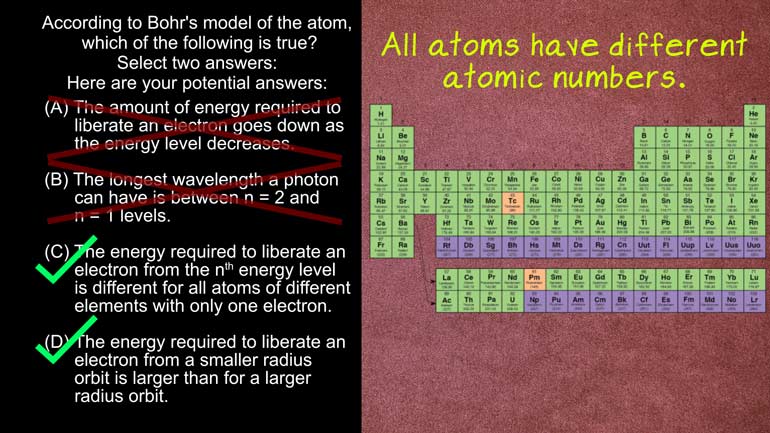
AP Physics 2: 1.5 Properties of Objects and Systems. According to the Bohr's model of the atom, which of the following are true?
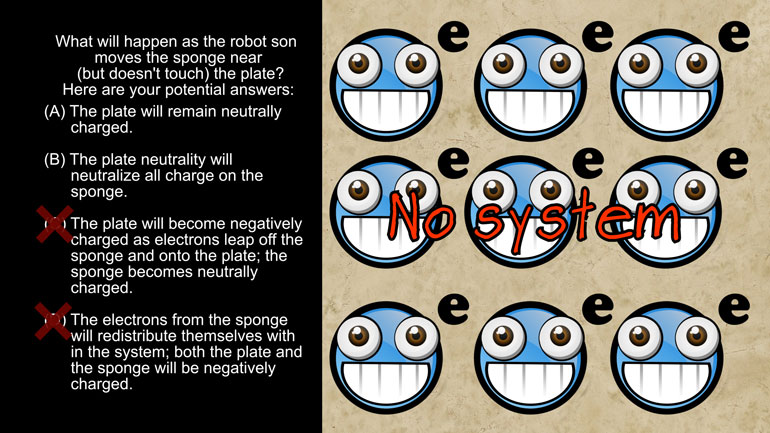
AP Physics 2: 2.2 Properties of Objects and Systems. What will happen as the robot son moves the sponge near (but doesn't touch) the plate?
AP Physics 2: 2.4 Properties of Objects and Systems. How could you show the carnival barker an emission spectrum?