The Chemistry of Heartburn and Antacids
Have you ever seen the commercials with guy from Cars? For some reason, he never learns and just keeps eating too many things that upset his stomach. Didn't his mom ever warn him about eating too much candy? Tummy aches all around. Even worse than eating too much candy, eating too much pizza or other foods can lead to heartburn.
 >
>
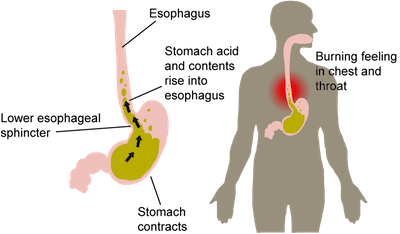
Heartburn is the worst.
The walls of the human stomach contain a gazillion cells that secrete acid in order to kill bad microorganisms and help in the digestion process.1 Heartburn occurs when these cells secrete extra acid (thanks to that extra half of a pizza) which spills over into the esophagus. Once in the esophagus, the acid causes a sensation of burning in the throat and chest. It feels a lot like someone is trying to barbeque inside your body. Gross.
One way to relieve this burning sensation is to neutralize the acid buildup with an antacid. You've probably heard of some of them: Tums, Rolaids, Alka Seltzer, Maalox, Milk of Magnesia are a few. Each of these over-the-counter drugs has an active ingredient with neutralizing power. The most common active ingredients are magnesium and aluminum hydroxides and bicarbonate or carbonate salts.2

Antacids make everything better. (Image from here.)
Let's get our chemistry on and figure out how antacids work their magic. The bicarbonate ion, HCO3-, reacts with the hydronium ion, written as H3O+ (from the acid buildup) to form a carbonic acid. This then undergoes a decomposition reaction to give carbon dioxide and water. We don't know about you, but we'd much rather have carbon dioxide and water in our stomach than extra acid. Many people even prefer a little carbonation from time to time. Here are the two reactions in equation form:
HCO3- (aq) + H3O+ (aq) → H2CO3 (aq)+ H2O (l)
H2CO3 (aq)→ CO2 (g)+ H2O (l)
The carbonate ion, CO32-, does its thang in a very similar manner with slightly different stoichiometry.
CO3-2 (aq) + H3O+ (aq) → H2CO3 (aq) + 2 H2O (l)
H2CO3 (aq)→ CO2 (g)+ H2O (l)
It's important to note that small quantities of these over-the-counter medications are A-Okay to ingest, but taking too much or too regularly can really mess up the other chemistry going on in the body. Anyone with heartburn for more than a few days at a time should talk to a doctor.