Alkaline Earth Metals
The Not So Terrible Twos: Alkaline Earth Metals
The second column of the periodic table is called group two. It's also called the alkaline earth metal family. Beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). Is that enough –iums for you?
Way back when, before Playstations, iPhones, automobiles, and even commercial electricity, scientists called "elements" that were non-metallic, water insoluble, and fire resistant, "Earths."5 In actuality, these "elements" were compounds composed of a metal and oxygen. Later these compounds were renamed oxides. The metal components of the oxides behaved differently, but still resembled the alkali family metals. After some quick brainstorming and mish-mashing of names, the two concepts were combined to create the Frankenstein-creature we now call alkaline earth metals.

Barium, an Alkaline Earth Metal.
This wholesome family lies in the s-block of the periodic table, which means their two valence electrons are in an s orbital. Here are a few alkaline earth metal electron configurations:
• Beryllium: [He]2s2
• Magnesium: [Ne]3s2
• Strontium: [Kr]5s2
The alkaline earths need to lose two measly electrons to achieve the super stable noble gas configuration. Piece of cake, right? Actually it is fairly easy for these elements to lose their valence electrons. The elements are not as reactive as their group one neighbors, but they're still very reactive.
Examples of Reactions Involving Alkaline Earth Metals:
Ca (s) + Cl2 (g) 2CaCl2 (s)
2Mg (s) + O2 (g) 2MgO (s)
Because of their high reactivity, the alkaline earth metals are not found in their elemental forms naturally. Instead they're found in the Earth's crust in their +2 oxidation state, which is the charge they achieve after losing the two valence electrons. To achieve this state, these elements can form halide complexes of the form MX2 (where M is the metal and X is the halide) or metal oxide complexes. Who knew the Earth's crust could be so interesting!
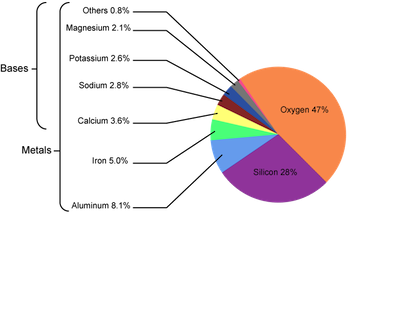
Composition of the earth's crust.
The metals of group two are harder and denser than the members of the group one family, but the hardness and density values are still relatively low compared to other metals of the periodic table. These metals are also silver-colored and soft. Not bunny rabbit type soft, but more fragile than the run-of-the-mill rock.



A few silver group two metals: magnesium, calcium, and barium.
Alkaline earth metals play a huge role in biology. Magnesium, for example, is absolutely essential to all organisms.6 It's a key component of chlorophyll, the green pigment in plants responsible for photosynthesis. Clearly, if someone wants to have a sun-energy harvesting superpower (Plant Man?), they should start taking their magnesium gummy vitamins.
When plants are deficient in magnesium their leaves begin to yellow, a process called chlorosis. Unless treated, chlorosis will result in a plant funeral.
Not a plant lover? Not concerned about photosynthesis? Well, magnesium plays a pivotal role in the human body as well. It's the second most abundant element inside human cells, and the fourth most abundant positively charged ion in the human body.7 Within the body's cells, Mg serves literally hundreds of functions.
How many times has your mom told you to drink your milk? "It's good for your bones!" Is mom right? Of course. One cup of milk has roughly 300 mg of calcium content.8 Calcium, as it turns out, is another essential alkaline earth metal. It helps keep our bones strong and our muscles functioning properly. Yet another reason to always listen to your mother.
Unlike its group two family members, Radium (Ra) is not quite so biologically friendly. It was discovered in 1898 by Pierre and Marie Curie.9 Ra is a bright silver radioactive metal that darkens upon exposure to air. Radioactive? This means that it emits energy in the form of rays, waves, or particles over time. The Curies spent so much time studying radioactivity that their handwritten laboratory notes are still too radioactive today for safe handling.9
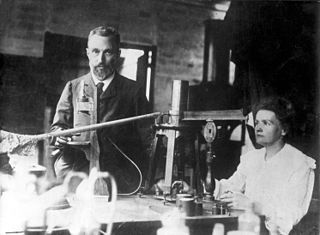
Pierre and Marie Curie working in the laboratory.