Periodic Table Trends
The Periodic Table is Oh So Trendy
At this point, we've examined various elemental occupants of the periodic table. Trust us, understanding general trends within families will come in handy one day, either on a test or when playing Jeopardy!. But that's not all the periodic table has to offer. The truth is, if you look at the table as a whole, some even more powerful trends start to emerge that can help us compare elemental properties and even predict reactivity. It's like having a flat, gigantic crystal ball.Atomic Radius
By definition, the atomic radius is one-half the distance between nuclei of two atoms. In other words, atomic radii are used to measure atomic size. In general, atomic size decreases as we move left to right across the periodic table. This can be a little confusing, but we have you covered—read on.As we move left to right across a period we are also observing an increase in atomic charge (the number of protons is increasing). This greater nuclear attraction pulls the electrons in more closely, and the atomic radii actually decrease. Think of it like a big nuclear hug. The more protons, the more love, and the electrons are pulled in closer to the nucleus.
Going down a group, atomic radii increase. This time, we're comparing electrons in different shells. Electrons in a 1s orbital are closer to the nucleus than electrons in a 2s orbital. In essence, the electrons in the outer shells don't "feel" the pull of the nucleus as strongly as those that are closer to the nucleus. Think about all the funky magnets you keep on your refrigerator. You use one of the magnets to hold up your prized study hall doodle. No problem—the magnet holds the single piece of paper with ease. But then you start adding more and more pieces of paper. (You've created some mad cool doodles this week.) Eventually, as you add more and more layers, the magnet loses its hold and falls off. This is similar to the idea of electron shielding. As we add more and more orbitals, the outer elections feel less of a pull from the nucleus and are able to get further away—hence the larger atomic radii as we go down the group.

Atomic radii trends in the periodic table.
Electronegativity
Electronegativity is the property describing an atom's ability to attract an electron. Electrons are drawn to elements with high electronegativites like Taylor Swift is drawn to boyfriends. Remember, elements are just dying to have a stable noble gas configuration. The elements that only need one or two extra electrons to get to that state are the most electronegative—they want electrons and they'll do anything to get them.As we move to the right across a period of elements, electronegativity increases. Atoms can either gain electrons or lose electrons. When the valence shell of an atom is less than half full, it's easier to lose an electron. (Those elements want to downsize, so they are practically giving electrons away.) When the valence shell of an atom is more than half full, it's easier to gain an electron; those elements will try to get a full shell by adding on.
As we move down a group, electronegativity decreases. As we navigate down a group the atoms get bigger and bigger with more and more electrons. This means the outermost electrons get further and further away from the positively charged nucleus. The consequence? Electronegativity decreases. Again, think of the magnet being shielded by all those doodle-pages.
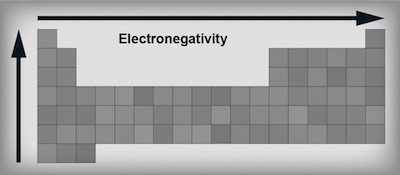
Electronegativity trends in the periodic table.
It's really important to remember these are just trends not rules. There are always exceptions. For example, the noble gases have negligible electronegativity values despite being on the very right hand side of the periodic table and the transition metals have only very small differences in their electronegativity values.
Knowing what we know, what is the most electronegative element?
Both arrows point to fluorine.
Ionization Energy
Ionization Energyis the amount of energy needed to remove an electron from an atom. Elements on the left hand side of the periodic table like alkali and alkaline earth metals have low ionization energies because losing an electron (or two) would make them achieve the noble gas configuration. Sometimes it's just easier to downsize to a lower shell, especially when you only have to lose a couple electrons to do it. Elements on the right hand side of the table have higher ionization energies because want more electrons, not less. Think of the elements on the right as being like Gollum and the golden ring represents electrons. Their electrons are precious, and it would take a lot of energy to take them away. Thus, ionization energies increase from left to right across the table—the left side is giving, the right side is Gollum.We've already figured out that as we go down a group the atoms get larger and the outermost electrons move further away from the positively charged nucleus. Which are stronger, long-distance relationships or short-distance relationships? As any rom-com fan will know, long-distance relationships rarely stick. The answer is the same for electrons. Electrons that are further away from the nucleus are not as tightly bound as electrons that are closer; therefore as we move down a group the ionization energy gets smaller.
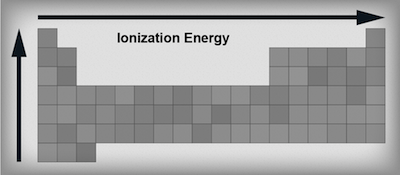
Ionization energy trends in the periodic table.
Electron Affinity
As the name suggests, electron affinity is the measure of how much an atom "wants" another electron. When an atom accepts an additional electron, it will either gain or lose energy. Electron affinity is a quantitative measure of that energy change.Electron affinity increases from left to right across the periodic table. This is caused by the decrease in atomic radius. As we already explained, moving from left to right across a period, atoms become smaller and smaller as the atomic number increases. This causes the electron to move closer to the nucleus, thus increasing the electron affinity.
Electron affinity decreases as we proceed down a group. This again is caused by the increase in atomic radius (there are more orbitals). When an electron is added to an atom with a large atomic radius, the electron is not strongly attracted to the distant nucleus. In contrast, when an electron is added to an atom with a small atomic radius, the electron is wickedly attracted to the very close nucleus.

Electron affinity trends in the periodic table.
The trends for electronegativity, ionization energy, and electron affinity are eerily similar. Remember that for your next quiz.