Metals, Metalloids, and Non-metals
Metals, Metalloids, & Non-Metals, Oh My!
It's hard to believe, but we've already navigated through a huge chunk of the periodic table. So far it's been pretty straightforward: explore a column here, learn about a column there, with elements of the same column sharing similar properties. Our next stop on this chemistry train 'o fun is the p-block. In this section, dividing elements into columns is so 2000 and late. Instead, the p-block is split into metals, metalloids, and non-metals.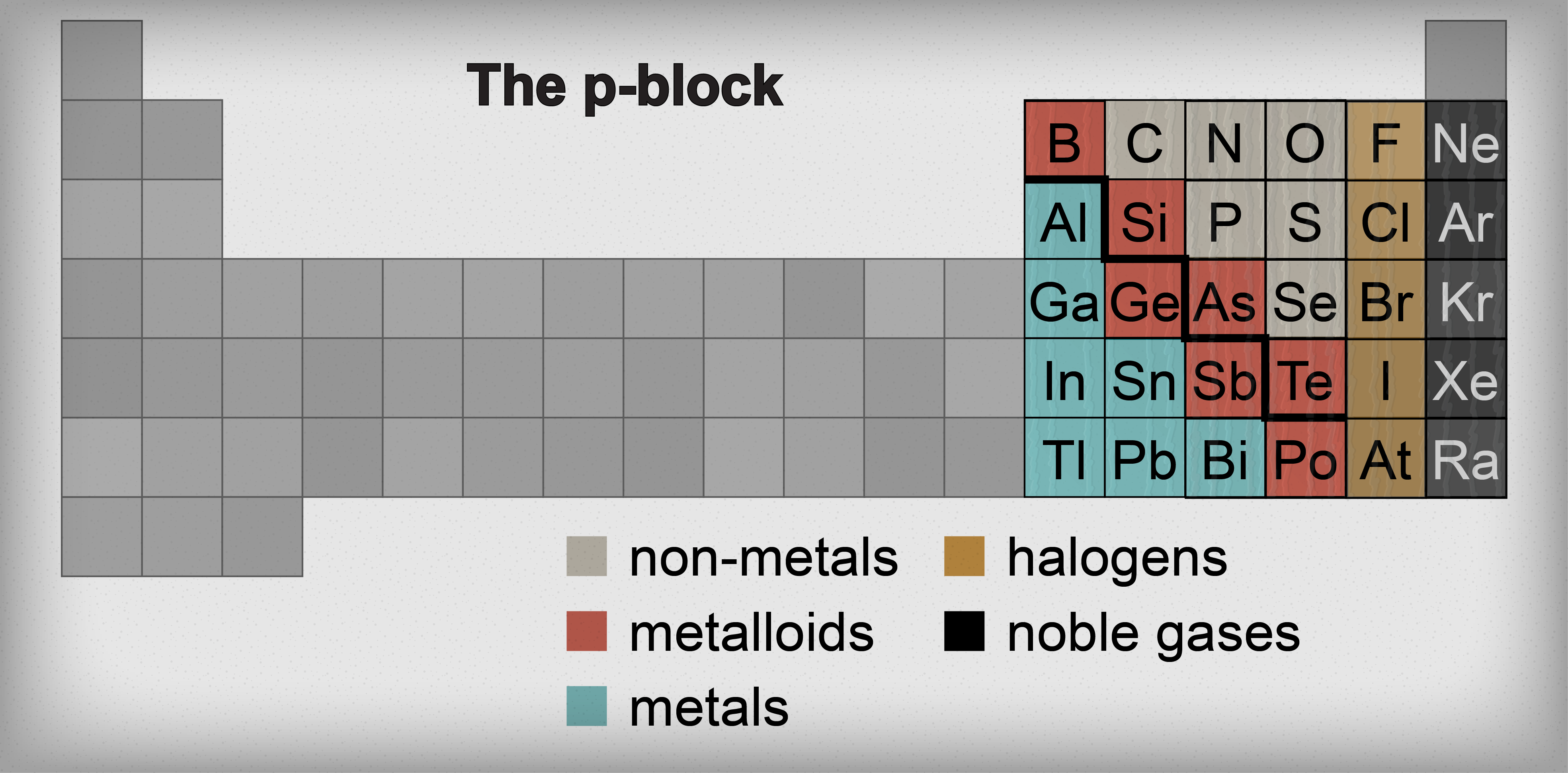
Metals, metalloids, and non-metals.
Have you ever wondered what that bold zigzag line is on the right side of the periodic table? Seems out of place. Think of it as the prime meridian of the periodic table. It divides the metals on the left half of the table from the non-metals on the right side of the table. Of course there are those pesky elements residing on the zigzag that don't quite fit in either category. Those elements are dubbed the metalloids.
The elements with the distinguished honor of being called a metalloid are boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), and polonium (Po). These elements have some properties that make them metal-like and other properties that make them non-metal-like. For this reason, these Frankenstein-type elements are also called semimetals or semiconductors.
The electronegativity and ionization energy values of metalloids lie in between those of metals and non-metals. This is the reason they possess some characteristics of metals and some characteristics of non-metals. Take silicon (Si), for example. It's shiny and silver like a typical metal, but it is brittle and a poor conductor of electricity. It's so confused.

Silicon: shiny and silver, but it's not a metal. It's a metalloid.
Okay, what about reactivity? Do metalloids react the same way as their metallic neighbors or their non-metallic neighbors? The truth is, it depends on the element with which they are reacting. Boron, for example, reacts like a metal when reacting with fluorine, but reacts like a non-metal when reacting with sodium.

Boron, a metalloid, turns green when put in a flame.
Metalloids play an important role in our everyday lives. Electricity and heat can travel through metalloids but not as easily as in true metals. This is why metalloids are often called semiconductors, particularly germanium and silicon. Can you guess what the main component of modern electronics, phones, computers, and radios are? That's right, semiconductors. We'll talk more about these fancy-pants devices later.

Metalloids (or semiconductors) are important components of electronic devices.
Metalloids have other uses as well. For example, antimony is used in alloys such as pewter and as a flame retardant in plastics. Boron is used as a bonding agent in magnets and other chemical substances. Bonding agent? This is a way of saying boron helps things stick together.

Boron is used as a bonding agent in pewter.
Enough about metalloids already—let's move on and discuss their next-door neighbors, the metals. That's right. The transition metals aren't the only metals on the table. The p-block has its own set of metals located to the left of the metalloids. These metals are aluminum (Al), gallium (Ga), indium (In), tin (Sn), thallium (Tl), lead (Pb), and bismuth (Bi).
These metals are solid, shiny, and good conductors of electricity and heat, all properties that we normally associate with metals. They are also ductile and malleable, which means they can be drawn into thin wires and hammered into thin sheets. Like their transition metal counterparts, these elements tend to lose (and therefore conduct) electrons fairly easily.
Aluminum is one of these metals. We're all familiar with good 'ole Al (Z = 13). Aluminum foil, anyone? It's silvery white in color and very reflective. Aluminum is used to build planes and buildings because it combines strongly with other elements making very tough and durable materials. It's also fairly non-toxic so we can wrap our burritos in it and fork our food with it and not get ill. We still wouldn't recommend eating aluminum. In high enough doses, it can kill you.
Need a study break? Cats love aluminum foil too. Check it out. This too.
Another p-block metal is lead (Pb, Z = 82). Its chemical symbol is derived from the Latin word plumbum, which means waterworks. Seems awfully random, but Pb has been used for ages to make water pipes. Even the ancient Romans used this soft, malleable, and corrosion resistant metal in their plumbing systems. Unfortunately lead can build up in the human body and cause serious health issues. Just ask the Mad Hatter.
To the right of the metalloid staircase is a whole new brand of elements…the non-metals. For now, we'll only discuss carbon (C), nitrogen (N), oxygen (O), phosphorus (P), sulfur (S), and selenium (Se). The last two rows of the periodic table, the halogens and the noble gases, are also non-metals but we'll cover those in the next two sections.
The properties of non-metals are the opposite for those of metals. Non-metals love electrons, especially the ones they already have, which means they have high ionization energies and high electronegativities. Their large electron affinities means they have the ability to gain electrons easily. You can call them electron hogs.

Non-metals have high electron affinities. They're electron hogs.
Unlike metals, non-metals are poor conductors of heat and electricity. They are brittle and usually dull in color. They sound boring, but they're actually the coolest.
Carbon (C, Z = 6) is one of the least boring elements on the periodic table. It's the sixth most abundant element on Earth and is the basic for organic chemistry; it occurs in all living organisms.11 Ever wonder what you and a slug have in common? Carbon.
Elemental carbon can exist as one of the hardest substances on Earth (diamonds) and as well as one of the softest substances on Earth (graphite). There are a gazillion uses of carbon known to humankind: pencils, lubricants, odor remover, radiocarbon dating… the list goes on.
An essential non-metal we're all familiar with is oxygen (O, Z = 8). Oxygen is the third most abundant element in the universe and accounts for 21% of Earth's atmosphere.11 It's a colorless, odorless, and tasteless gas, but is essential for human life. In its solid and liquid phase, oxygen is pale blue, strongly paramagnetic (basically, it's magnetic...), and explosive. Who knew oxygen was so dangerous?
The last non-metal we'll chat about is sulfur (S, Z = 16). It's a pale-yellow, odorless, and brittle solid. Some of the complexes it forms, however, like gaseous H2S are super smelly. If you ever get a chance to smell H2S, don't. It smells like rotten eggs.