ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Science Practice 2 Videos 10 videos
AP Physics 2: 2.2 Changes and Conservation Laws. Which of the following is not one of the possible energy levels for double-ionized lithium?
AP Physics 2: 1.3 Changes and Conservation Laws. To what temperature does the aluminum sheet need to be heated in order for the square peg to fit t...
AP Physics 2: 1.3 Fields in Space. How long will it take PANCAKE to reach the plate?
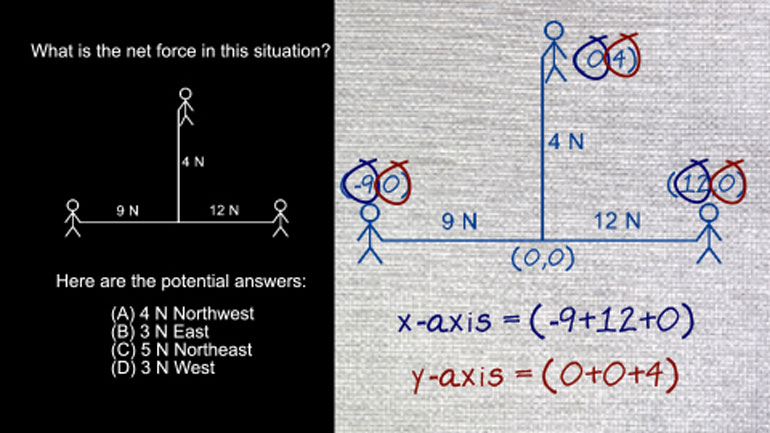
AP Physics 2: 1.4 Object Interaction and Forces 5 Views
Share It!
Description:
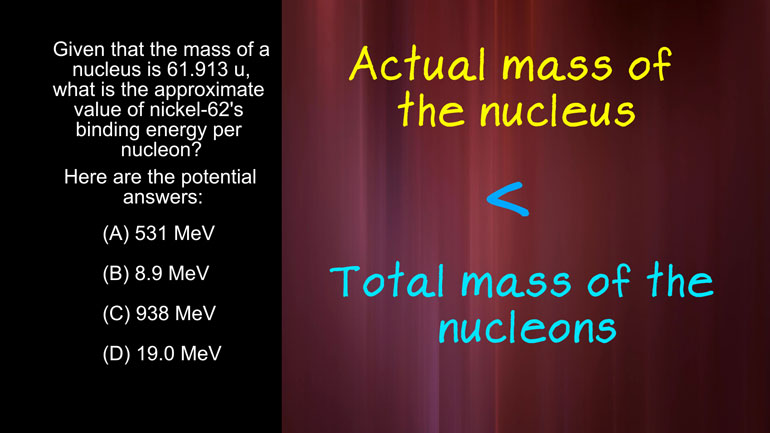
AP Physics 2: 1.4 Object Interaction and Forces. What is the approximate value of nickel-62's binding energy per nucleon?
Transcript
- 00:04
Here's your shmoop du jour brought to you by prizefights [Men in boxing ring]
- 00:07
which create a real dilemma for us because while we hate fighting but we
- 00:10
love prizes so if we need to we'll punch a box of Cracker Jacks or just get that [Girl punches box of cracker jacks]
- 00:15
price and don't you think we want our heavyweight prizefight for most stable
- 00:20
nucleus has been highly contested with nickel 62 and iron 56 duking it out as
Full Transcript
- 00:27
the top two contenders while it's true that iron 56 has the lowest mass per [Iron 56 and nickel 62 in a boxing ring]
- 00:31
nucleon nickel 62 with the highest value of binding energy per nucleon of any
- 00:37
nuclei has ultimately been declared the winner well given that the mass of a
- 00:42
nucleus is 61 point 9 13 you what is the approximate value of nickel 62 s binding
- 00:49
energy per nucleon and more essential answers....
- 00:55
alright well we know that particles with like charges repel each other but in an [Particles with positive charge]
- 01:00
atom's nucleus there are protons which all have a positive charge and neutrons
- 01:05
well which don't have any charge why are they all together it's because of the
- 01:08
strong force that's what binds nucleons and allows all the different elements in
- 01:13
the universe to form without the strong force there would only be hydrogen atoms [Hydrogen atoms at a bar]
- 01:18
which would make the universe pretty boring in order to hold the particles
- 01:21
together the strong force converts some of the mass of the nucleons into energy
- 01:26
well this means that the actual mass of the nucleus is less than the total mass
- 01:31
of the nucleons the difference between the expected mass and the real mass is
- 01:36
called the mass defect enough that we're saying nuclear defective it's nothing [Atoms appear angry on side street]
- 01:40
personal Einstein gave us the equation to calculate how much energy is created
- 01:44
by this loss of mass and yet it's the most famous equation in the world e
- 01:49
equals MC squared it means that energy equals mass times the speed of light
- 01:53
squared so it doesn't take much mass to create a whole lot of energy to answer [Dynamite explodes on road]
- 01:59
this question first we need to figure out how much mass is missing well nickel
- 02:03
has an atomic number of 28 meaning there are 28 protons in [Nickel electron shells appear]
- 02:08
each proton has a mass of 1.00 732 atomic mass units well since this
- 02:14
isotope has a mass number of 62 we can find the number of neutrons by
- 02:19
subtracting the protons from the mass number well neutrons are just a little [Neutron appears]
- 02:23
bit chubbier than their proton cousins they have a mass of 1.00 867 you /
- 02:29
neutron so they add up to 34.295 you got it all right when we add the
- 02:34
protons and neutrons we find an expected mass of 62.5 you to find the mass defect
- 02:41
we just subtract the actual mass from the expected mask that now we can
- 02:46
convert the mass to energy one atomic mass unit equals 931 mega electron volts [Atomic mass unit appears]
- 02:53
we multiply the mass defect by 931 math to find a binding energy of 540 9.29 med
- 03:01
alright well now let's take a look back at the question because by now we've [Man looking through paper]
- 03:04
pretty much forgotten why we're doing all this oh yeah we need to find the
- 03:08
binding energy per nucleon well there are 62 nucleons so we divide the binding
- 03:12
energy by that number the binding energy per nucleon is about 8.9 mega electron [Binding energy equation appears]
- 03:17
volts so the correct answer is B the strong force only acts on a very tiny
- 03:22
scale that it's the strongest force in the universe which makes sense as we saw
- 03:26
here a tiny amount of mass creates a large amount of energy and the strong
- 03:30
force is able to completely overcome the electromagnetic force that pushes the
- 03:34
protons away from each other and remember that violence is never the [Man trying to open snack pack]
- 03:38
answer unless our snack won't open then we might just lose it
Related Videos
AP Physics 2: 1.1 Properties of Objects and Systems. What is the magnitude and direction of the conventional current in this wire?
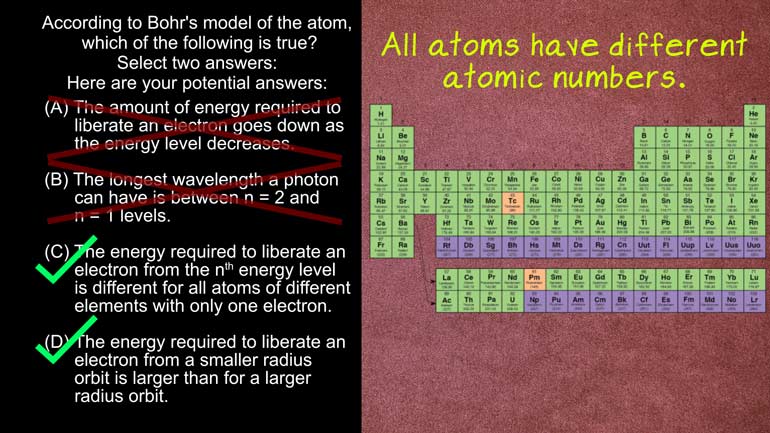
AP Physics 2: 1.5 Properties of Objects and Systems. According to the Bohr's model of the atom, which of the following are true?
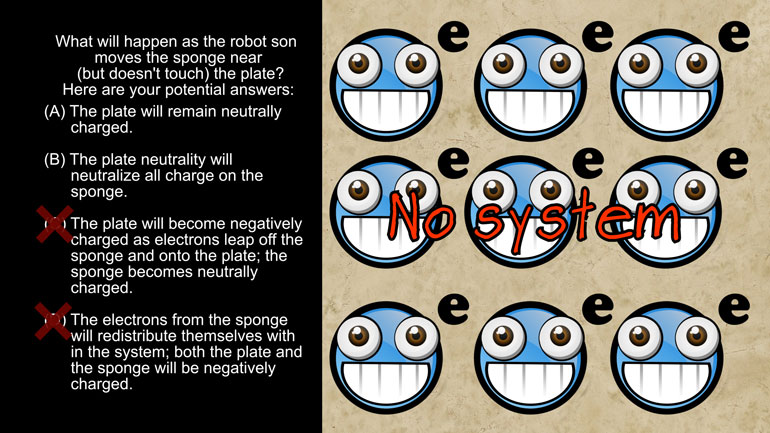
AP Physics 2: 2.2 Properties of Objects and Systems. What will happen as the robot son moves the sponge near (but doesn't touch) the plate?
AP Physics 2: 2.4 Properties of Objects and Systems. How could you show the carnival barker an emission spectrum?