ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
ASVAB Videos 144 videos
ASVAB Paragraph Comprehension 1.2 Summary. Which of the following best describes the purpose of this passage?
ASVAB Paragraph Comprehension 1.2 Vocabulary-In-Context. In this passage, the word "illustrious" most nearly means...what?
ASVAB Paragraph Comprehension 1.3 Vocabulary-In-Context. The word "preposterous" most nearly means what?
ASVAB Physical Science 3.2 170 Views
Share It!
Description:
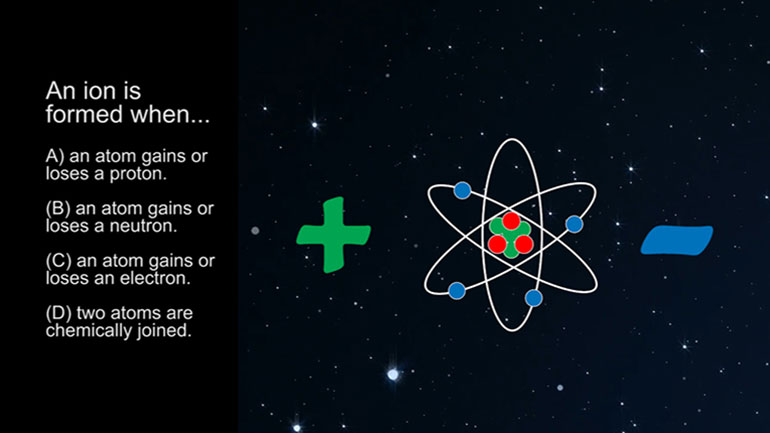
ASVAB Physical Science 3.2. A solution with equal concentrations of H+ ions and OH- ions should have a pH of...what?
Transcript
- 00:00
[ musical flourish ]
- 00:03
And here's your Shmoop du jour, brought to you by bases,
- 00:06
which are vital in any good game of hide and seek.
- 00:09
And they're important to science, too. Just not as fun.
- 00:12
All right. A solution with equal concentrations of hydrogen ions
Full Transcript
- 00:16
and hydroxide ions should have a pH of what?
- 00:20
pH, not "fff."
- 00:23
And here are the potential answers.
- 00:25
All right, so what's going on here? Well, let's see.
- 00:28
We've got hydrogen ions, or H+,
- 00:30
hydroxide ions, or OH-,
- 00:34
and "fff" level. pH level.
- 00:36
All right, well, that's great. What does it all mean?
- 00:40
We're talking about our old friends acids and bases.
- 00:43
Or at least that's how we like to think of 'em.
- 00:44
Acids are chemicals that have a lot of hydrogen ions,
- 00:47
symbolized by H+, and bases are chemicals that have a lot of hydroxide ions,
- 00:52
symbolized by OH-, like that.
- 00:55
The pH scale is a measurement of the concentration
- 00:59
of these ions. And it runs from zero to 14.
- 01:02
The lower the number, the more acidic the substance is,
- 01:06
meaning it has more hydrogen ions. The higher the number,
- 01:09
the more base the substance is, meaning it has more hydroxide ions.
- 01:13
Got it? Well, if there is an
- 01:16
equal concentration of hydrogen and hydroxide ions,
- 01:20
then the pH level would be seven, since that's
- 01:23
right in the middle of the scale. So option C - seven is the correct answer.
- 01:27
Now that we know the answer, we can move on to more important matters,
- 01:30
by which we mean ready or not, here we come.
Related Videos
ASVAB Paragraph Comprehension 1.2 Summary. Which of the following best describes the purpose of this passage?
ASVAB Paragraph Comprehension 1.2 Vocabulary-In-Context. In this passage, the word "illustrious" most nearly means...what?
ASVAB Paragraph Comprehension 1.3 Vocabulary-In-Context. The word "preposterous" most nearly means what?
ASVAB Paragraph Comprehension 2.3 Summary. Which of the following best describes the main idea of this passage?
ASVAB Word Knowledge: Word Roots, Prefixes, and Suffixes Drill 1, Problem 1. Which of these words is closest in meaning to incoherent?