ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Science Videos 686 videos
In this video, we dive beneath the sea to review the kinds of interesting animals that live in the deep blue.
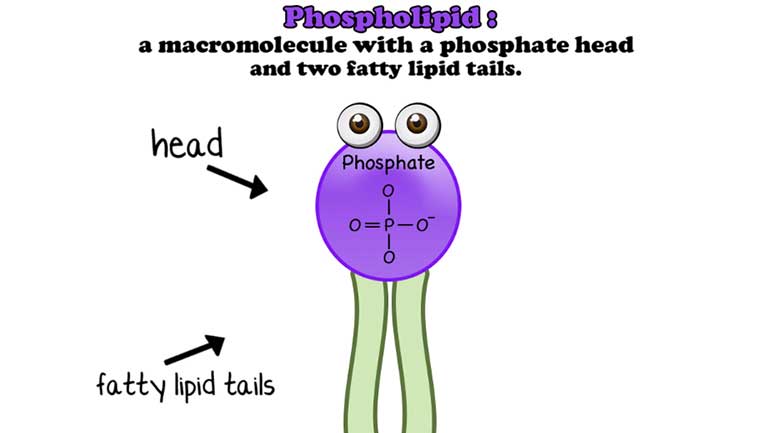
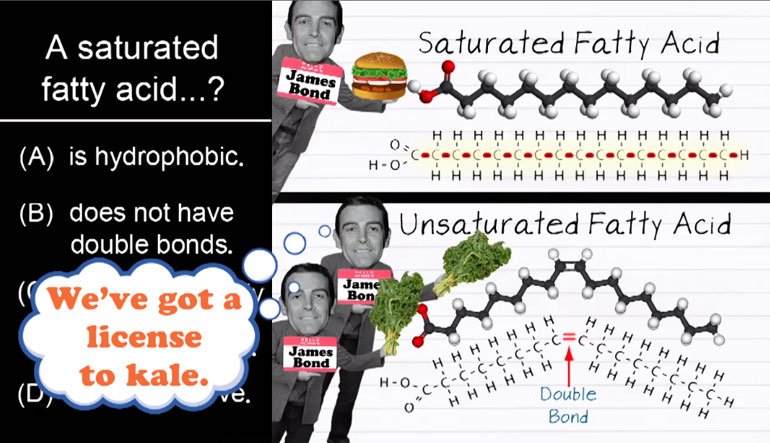
Anything that has a cell (bacteria, listen up!) has phospholipids that keep the cell contained and give it form and shape. Phospholipids protect us...
Chemistry: 4.5 Quantum Mechanical Model 66 Views
Share It!
Description:
They're kind of like regular models, except instead of clothes they show off electrons. We don't advise any people making the same switch before going out in public, though. We don't imagine that would go over too well.
Transcript
- 00:03
The Schrodinger family
- 00:05
has turned out a ton of successful models. [Schrodinger family together]
- 00:07
There's Emmy Schrodinger, who models
- 00:10
runway fashions in Paris, [Emmy falls off fashion runway]
- 00:13
Candy Schrodinger, who is regularly featured on
Full Transcript
- 00:15
the covers of magazines like Vogue and
- 00:17
Cosmo, and Beatrice Schrodinger, who's an [Beatrice waving]
- 00:21
up-and-coming hand model. She's thinking
- 00:23
of branching into feet. But none of
- 00:25
that's good enough for Delia Schrodinger,
- 00:27
the brainy one of the bunch. She's [Delia reading Science book]
- 00:30
determined to be a quantum mechanical
- 00:31
model. When her sisters asked her what in
- 00:34
the world that is, well, she explains, a
- 00:36
quantum mechanical model is a visual
- 00:39
demonstration of the location of an atom's
- 00:41
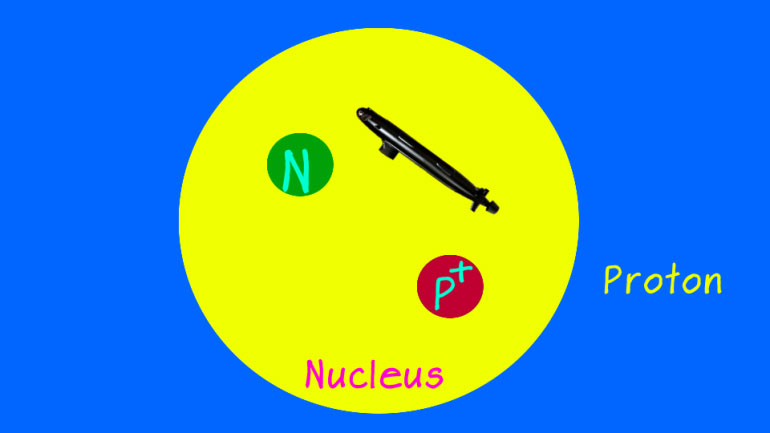
electrons. Basically, by dividing the [Electrons orbiting an atom]
- 00:44
space around a nucleus into orbitals, it
- 00:47
offers probable locations where an
- 00:49
electron might be found, which is usually
- 00:52
about where she loses them. Well, Delia
- 00:54
will not be deterred from her dream. Delia knows that to become the best [Delia inside an atom]
- 00:58
quantum mechanical model she can be,
- 01:00
she's going to need her orbitals
- 01:02
categorized by quantum number. For
- 01:05
starters, she's got her principal quantum
- 01:07
number, symbolized by the letter n,
- 01:10
because the letter n is in the word
- 01:12
principal somewhere... right there. This number [Hand points to Principal]
- 01:15
represents the energy level containing
- 01:17
an electron and ranges from 1 to 7. The
- 01:20
lower the number, the lower the energy.
- 01:22
If the principal quantum number is six
- 01:24
or seven on the other hand, then the
- 01:26
electron has more energy than a [Man holding dog bowl and dog follows]
- 01:27
chihuahua at feeding time. Delia's next
- 01:30
orbital is categorized by the orbital
- 01:32
quantum number, or angular momentum
- 01:35
quantum number, symbolized by the letter
- 01:37
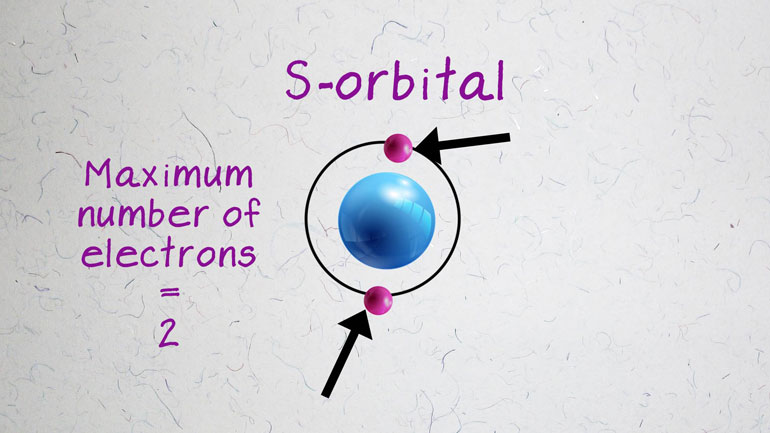
l. This number indicates the shape of the
- 01:40
electron cloud, as well as the number of
- 01:43
sublevels of energy present. There are
- 01:46
two sublevels of energy in energy
- 01:47
level two, three sublevels of energy in
- 01:50
energy level three, and so on. Well, the
- 01:52
third number is the magnetic quantum
- 01:54
number, symbolized by the letter m, which
- 01:57
explains how the orbital is oriented. And, [Orbitals talking to each other]
- 02:00
it further breaks down the previous quantum
- 02:02
number. It's almost like the principal
- 02:04
quantum number is a book, the angular
- 02:06
momentum quantum number is a chapter, and
- 02:08
the magnetic quantum number
- 02:10
is a page, to put things in terms a
- 02:13
literature nut can understand. Finally
- 02:15
there's the spin quantum number,
- 02:17
symbolized by the letter s. Well, all this
- 02:20
one does is demonstrate what direction [Atom orbitals spinning in different directions]
- 02:22
an electron is moving in an orbital,
- 02:24
clockwise or counterclockwise. And yeah,
- 02:26
electrons are always moving... not really
- 02:28
the "Netflix and chill" kind of type. Well, [Electron relaxing watching TV]
- 02:32
Delia's sisters may not be able to
- 02:33
understand her fascination with
- 02:35
electrons, but at least they support her
- 02:36
in her efforts. There was actually a halfway
- 02:39
decent turnout at her last subatomic [Delia modelling as an atom and walks down runway]
- 02:41
particle show.
Related Videos
When you're about to marry the love of your life, not many things could stop you. However, finding out that your future hubby is keeping his crazy...
Here at Shmoop, we work for kids, not just the bottom line. Founded by David Siminoff and his wife Ellen Siminoff, Shmoop was originally conceived...
ACT Math: Elementary Algebra Drill 4, Problem 5. What is the solution to the problem shown?
AP® English Literature and Composition Passage Drill 1, Problem 1. Which literary device is used in lines 31 to 37?
AP® English Literature and Composition Passage Drill 2, Problem 1. What claim does Bacon make that contradicts the maxim "Whatsoever is delig...