ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Science Videos 686 videos
In this video, we dive beneath the sea to review the kinds of interesting animals that live in the deep blue.
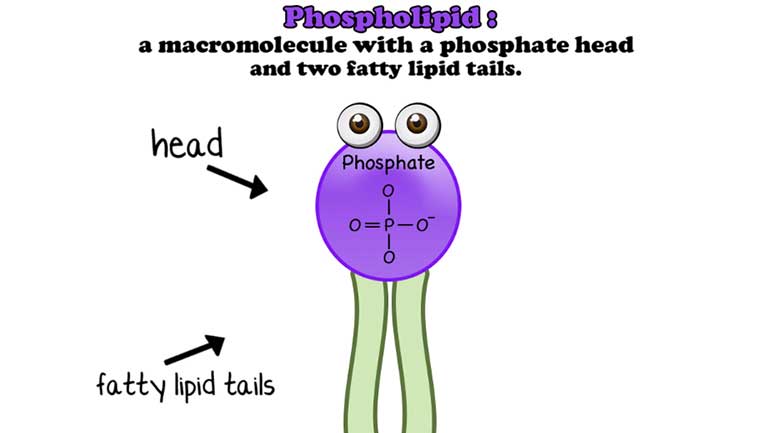
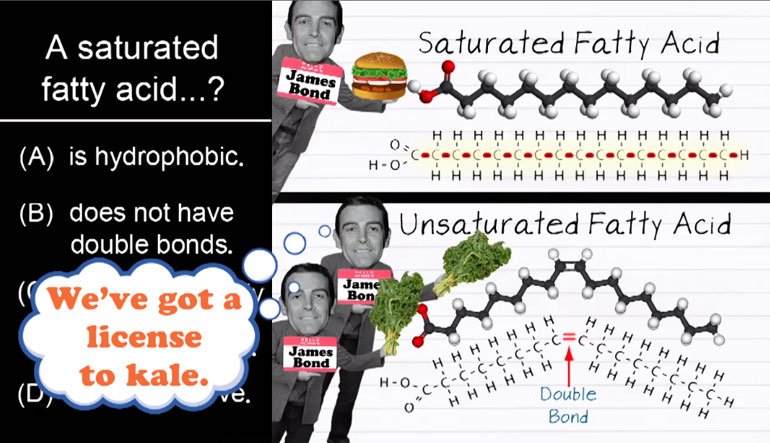
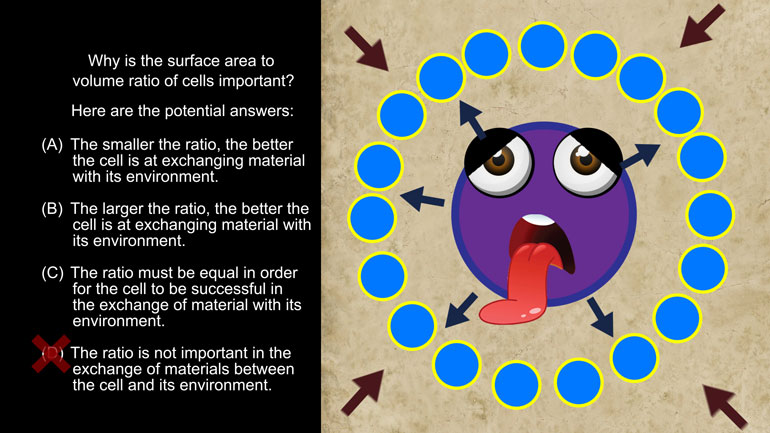
Anything that has a cell (bacteria, listen up!) has phospholipids that keep the cell contained and give it form and shape. Phospholipids protect us...
Chemistry: 5.1 History of the Periodic Table 120 Views
Share It!
Description:
Periodically throughout your life, you're going to have to learn about the periodic table. Today's lesson about history and nothing but history. Period.
Transcript
- 00:04
When it comes to learning about the origins of the periodic table...
- 00:08
...there's just one thing you need to remember.
- 00:10
The three M's.
- 00:12
John Mewlands, Dmitri Mendeleev, and Henry Moseley.
- 00:17
Okay, it's actually John Newlands. But two N's and an M is an awful mnemonic device.
Full Transcript
- 00:23
So let's start with John Newlands.
- 00:26
Oh, suck it up John. Your achievements are the important thing here.
- 00:29
A few years before the period table was compiled...
- 00:32
Newlands noticed that there was something special about the number seven.
- 00:36
And no, he wasn't a big gambler.
- 00:39
He realized that there were some uncanny similarities between certain elements.
- 00:43
All of which had atomic weights that differed by seven.
- 00:47
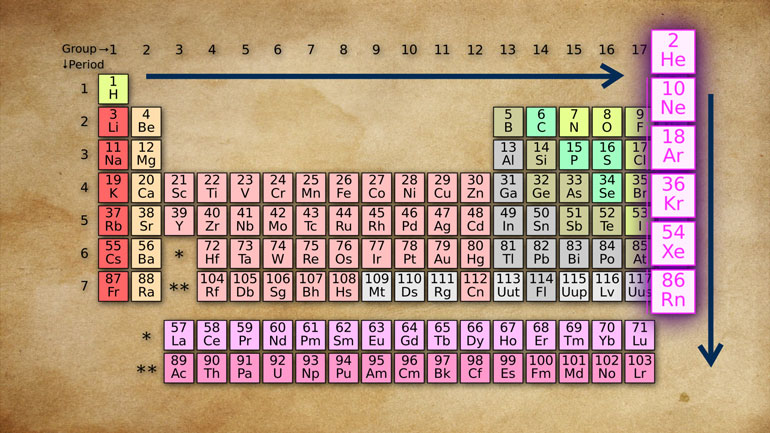
He organized the elements using what he called the Law of Octaves.
- 00:50
Because the way he sorted them looked a bit like octaves of music.
- 00:53
But it didn't sound nearly as pretty. Even when Newlands would attempt to sing about it in the shower.
- 01:00
It wasn't until 1869 that Dmitri Mendeleev...
- 01:02
Oh come on John, your part of the story's over now. Move on.
- 01:09
It wasn't until 1869 that Mendeleev was playing around with an organization of the elements himself.
- 01:14
He discovered that when you order them by increasing atomic weight, a pattern seemed to emerge.
- 01:20
It was always a reactive non-metal, then a very reactive light metal, then a less reactive light metal, and so on.
- 01:27
He plugged everything into the format we see today.
- 01:30
Although, not before experimenting with having all like elements in rows, rather than columns.
- 01:35
Excel spreadsheets probably would have tortured this guy.
- 01:38
Perhaps the best thing Mendeleev did was to leave spaces for undiscovered elements.
- 01:44
He'd figure out where there was a missing link and he didn't attempt to stuff something in there that didn't belong.
- 01:50
By recognizing roughly what should belong in those empty slots...
- 01:53
Mendeleev was actually able to predict the properties of some of those elements.
- 01:59
We bet Mr. Newlands would have been awfully jealous.
- 02:04
Okay, let's wrap this thing up.
- 02:06
The other big contributor to the periodic table was Henry Moseley.
- 02:10
Mendeleev still had a couple of question marks on his period table...
- 02:14
...and it was Moseley who was able to turn those question marks into exclamation points.
- 02:19
...Well, figuratively speaking.
- 02:21
After using an X-ray gun to measure the wavelengths, or X-rays...
- 02:25
Moseley performed a few nifty calculations and concluded that...
- 02:30
...there was an exact correlation between frequency and atomic number.
- 02:34
So the few spots that had given Mendeleev a headache finally had an answer.
- 02:39
Moseley had found the reason why organizing the elements by atomic number...
- 02:43
...didn't always work out exactly the same as organizing them by atomic mass.
- 02:46
Even though the modern periodic table is organized by atomic number...because it's pretty darn close.
- 02:52
Unfortunately, Mendeleev was dead by the time Moseley made his big breakthrough,
- 02:56
so he never got news of the discovery.
- 02:59
Huh, you have a really hard time letting things go, don't you there?
Related Videos
When you're about to marry the love of your life, not many things could stop you. However, finding out that your future hubby is keeping his crazy...
Here at Shmoop, we work for kids, not just the bottom line. Founded by David Siminoff and his wife Ellen Siminoff, Shmoop was originally conceived...
ACT Math: Elementary Algebra Drill 4, Problem 5. What is the solution to the problem shown?
AP® English Literature and Composition Passage Drill 1, Problem 1. Which literary device is used in lines 31 to 37?
AP® English Literature and Composition Passage Drill 2, Problem 1. What claim does Bacon make that contradicts the maxim "Whatsoever is delig...