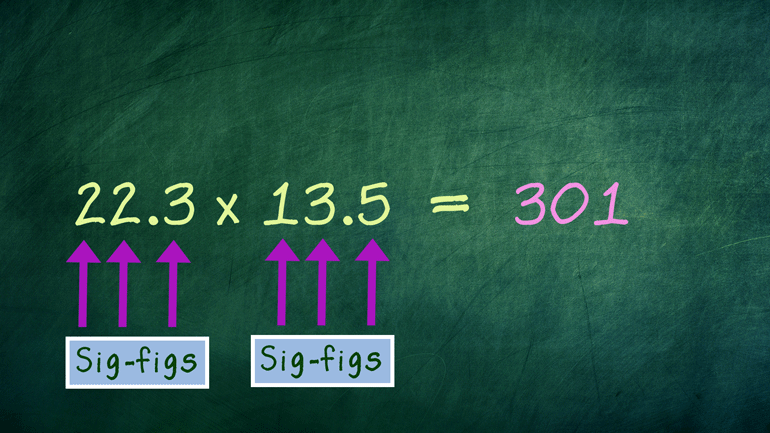ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Chemistry Videos 44 videos
Don't even think about stepping foot into a lab until you drink a nice big cup of safe-ty. Safety lesson number one: do not listen to this descript...
Today we're playing with fire. Wait, we're not supposed to say playing...having fun with fire? Today's lesson is on the colors that can be emitted...
These figures may not be significant to you, but they matter to us, okay? Oh, and to Science. They matter a ton to Science.
Chemistry: 2.1 Significant Figures and Scientific Notation 415 Views
Share It!
Description:
These figures may not be significant to you, but they matter to us, okay? Oh, and to Science. They matter a ton to Science.
Transcript
- 00:00
sweetie didn't before we get into this video we'd like you to start by writing
- 00:07
down all the digits of pi no writing okay we're getting a couple more things [Man jotting down notes in class]
- 00:12
all right so here's the problem with our request pi has an infinite number of
- 00:16
digits so if you were to actually write out all of its digits you'd never be [Man writing digits and gollum appears]
- 00:20
done fortunately the rule of significant
Full Transcript
- 00:23
figures lets us take a bit of a shortcut and only requires that we write down a [Man takes significant figure shortcut]
- 00:28
certain number of digits spending on a situation if we were performing a
- 00:32
calculation our answer is limited to the number of significant figures or sig [Girl using calculator]
- 00:38
figs as the cool kids column at reset in our unique precise number in other words
- 00:43
if we're multiplying 22.3 and 13.5 we're only going to include one two three sig
- 00:49
figs in our solution the other situation is when we're reading instrumentation in [Scientist reading instrument measurement]
- 00:54
which case the number of sig figs depends on the precision of the
- 00:58
instrument we're using the measure for now let's take a closer look at the
- 01:01
rules for calculation rule number one all nonzero digits are significant so if [Rule number one appears]
- 01:06
you've performed a calculation and your result is 114 write down 114 not 109 110
- 01:12
or no zeroes so smille rounding if you get a number that goes through the tens
- 01:16
place like 114 point 8 you write one 14.8 in this case you'd have four [Hand points to 114.8 number]
- 01:22
significant figures rule number two zeros between nonzero
- 01:27
digits are significant so if you get 104 well you still got three significant [Person shows three fingers]
- 01:32
figures even though one of them is a zero but as long as you've got some form
- 01:36
of a zero sandwich it counts rule number three trailing zeros zeros to the right [Girl takes bite of zero sandwich]
- 01:42
of a decimal point are significant so if your calculation comes out to one
- 01:46
hundred point zero degrees on the button you'd have four significant figures to
- 01:52
deal with all right finally rule number four leading zeros zeros to the left of
- 01:57
the first nonzero digits are not significant so say you come up with an [Boy appears with magnifying glass]
- 02:02
easy peazy number like 0.001 eight well one and the eight are significant the
- 02:10
zeros are not but we can't just write 18 correct
- 02:16
that's not the same thing as 0.0018 oh how right you are scientific notation is
- 02:21
basically just a shorthand way of writing out troublesome numbers in a way [Man writing out numbers in notepad]
- 02:25
that's easy to understand and won't give us carpal tunnel trying to write it for
- 02:30
0.0018 you write the first non-zero number we come across the one and make
- 02:36
it 1.8 times 10 to the negative third because we'd have to move the decimal
- 02:42
point three places to the right in order to get our 1.8 number now if something [Decimal points moves to the right]
- 02:48
had a measurement of zero point zero zero zero zero zero zero 95 - yikes we'd
- 02:54
write it as nine point five two times ten to the negative seventh and so on we
- 03:00
can use the scientific notation for numbers greater than zero - but when we
- 03:03
do the exponent is always positive like five hundred would be five times 10
- 03:09
squared Y squared well because we'd have to move the [Decimal points moves two places to the left]
- 03:13
decimal point two places to the left to get our five five hundred thousand would
- 03:18
be five times ten to the fifth and numbers greater than zero containing
- 03:23
decimals work to one thousand three hundred eighty eight point three would
- 03:27
be one point three eight eight three times ten to the third six point two
- 03:32
would be six point two times ten to the zero power switch is just one so yet the
- 03:37
zero power thing is also what Superman has when wearing a kryptonite necklace [Superman sitting on sofa with kryptonite necklace]
- 03:42
and that's it hopefully now you feel 1 times 10 squared percent smarter than
- 03:46
when we started
Related Videos
When you're about to marry the love of your life, not many things could stop you. However, finding out that your future hubby is keeping his crazy...
Here at Shmoop, we work for kids, not just the bottom line. Founded by David Siminoff and his wife Ellen Siminoff, Shmoop was originally conceived...
ACT Math: Elementary Algebra Drill 4, Problem 5. What is the solution to the problem shown?
AP® English Literature and Composition Passage Drill 1, Problem 1. Which literary device is used in lines 31 to 37?
AP® English Literature and Composition Passage Drill 2, Problem 1. What claim does Bacon make that contradicts the maxim "Whatsoever is delig...