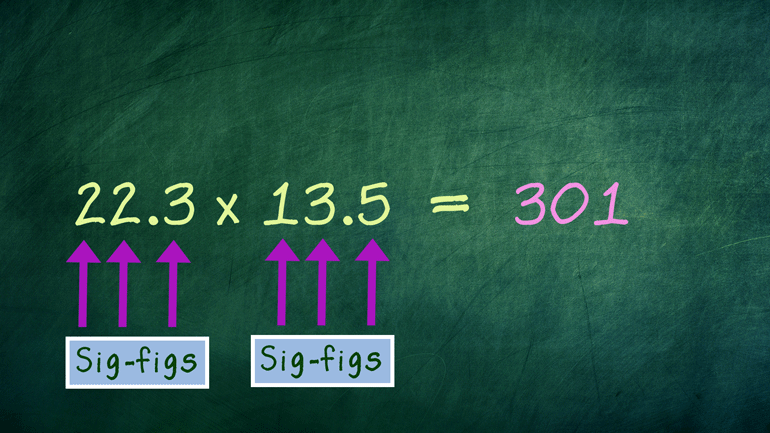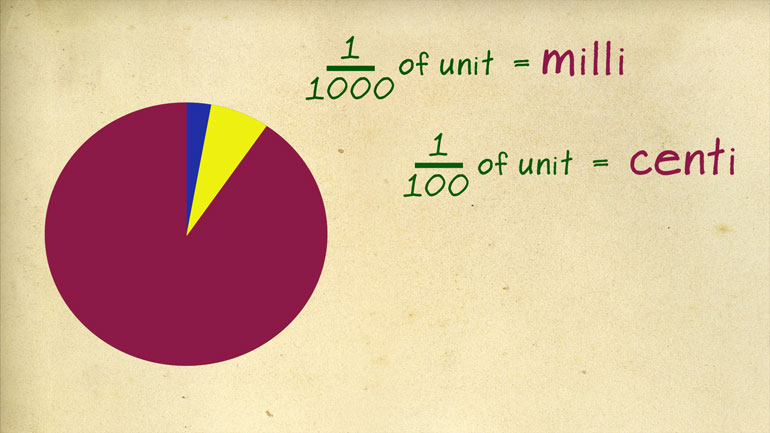ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Chemistry Videos 44 videos
Don't even think about stepping foot into a lab until you drink a nice big cup of safe-ty. Safety lesson number one: do not listen to this descript...
Today we're playing with fire. Wait, we're not supposed to say playing...having fun with fire? Today's lesson is on the colors that can be emitted...
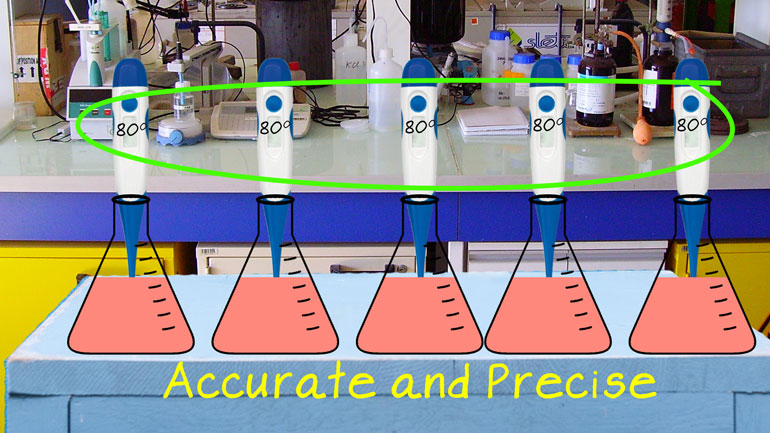
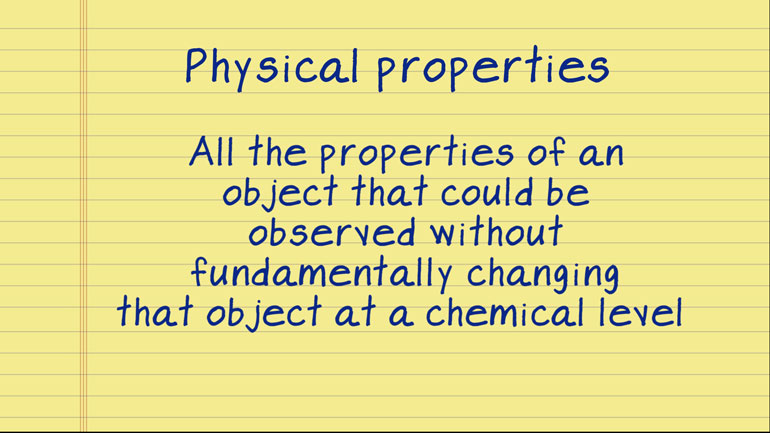
These figures may not be significant to you, but they matter to us, okay? Oh, and to Science. They matter a ton to Science.
Chemistry: 5.5 Metals in Transition 80 Views
Share It!
Description:
Today we're learning about how forgetful transition metals can be. They lose electrons just about as quick as they find them. They'd probably lose their nucleus if it wasn't mashed into their center.
Transcript
- 00:03
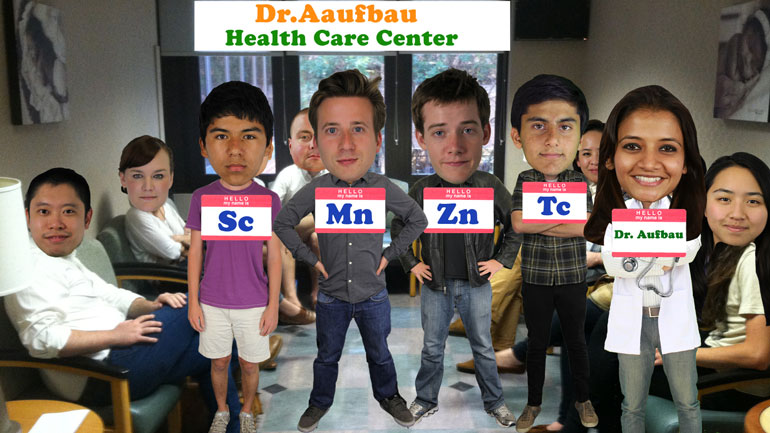
It's a little crowded in the doctor's [People sitting in doctors waiting room]
- 00:06
office today.
- 00:07
Every last one of the transition metals
- 00:09
is here to see Dr. Aufbau. [Transition metals standing in doctors office]
- 00:12
Yep, they're hoping to get some
Full Transcript
- 00:13
medication to help them with their acute
- 00:15
cases of claustrophobia. Taking a gander
- 00:18
at the periodic table, it's no wonder these [Periodic table of transition metals]
- 00:20
guys are feeling a bit, uh, boxed in.
- 00:23
They've got clowns to the left of them,
- 00:25
jokers to the right. Well, they've got some
- 00:27
fundamentally different elements on both
- 00:28
sides of them anyway... But why are they
- 00:31
all lumped together?
- 00:32
What's with the complete disregard for
- 00:34
one's personal space bubble, hmm?
- 00:36
Well, the transition metals, all these [Table of transition metals]
- 00:39
metals located from groups three to
- 00:41
twelve, are rock-hard metals. None of that
- 00:44
wussy, cut-through-it-with-a-butter-knife
- 00:46
lithium business.
- 00:47
Yeah, they're often combined to make
- 00:49
alloys like brass, which is a mixture of [Alloy lands on table]
- 00:52
copper and zinc, but we wouldn't
- 00:54
recommend you using that tidbit to make
- 00:56
fun of any of the other kids in the band.
- 00:58
Woodwind isn't an element either. [Two kids holding musical instruments]
- 01:00
All right, well these fellas often find
- 01:03
their way into jewelry because they
- 01:04
don't break down easily and they won't [Zinc and Gold necklace around a womans neck]
- 01:07
react with water. Otherwise, running
- 01:09
through a sprinkler with a neck full of
- 01:10
bling could be catastrophic. Whew, just the [Girl runs through sprinkler]
- 01:13
thought of that happening is terrifying.
- 01:14
No wonder some of these elements are losing it upstairs.
- 01:17
Well, the doctor finally calls all the [Doctor standing in her office]
- 01:19
metals in her office--yep, all at once.
- 01:20
It's a big office, so what do you want?
- 01:23
She tries to calm them all down by
- 01:24
explaining how special they are. In [Doctor waves her arm at the metals]
- 01:27
particular, she gushes about their
- 01:28
electrons. The transition metals are
- 01:30
located in the d-block, meaning their
- 01:33
outermost electrons reside in the
- 01:35
d-orbitals. Because these electrons are [Electrons of a metal atom]
- 01:38
so far away from their respective nuclei,
- 01:40
the attraction holding them in place
- 01:41
isn't very strong, which paves the way
- 01:44
for high electrical and heat
- 01:45
conductivity and allows the metals to be
- 01:48
malleable, which may not sound like such
- 01:51
a tremendous compliment to you, but trust
- 01:52
us: whisper some of those sweet nothings [Transition metals in the doctors office]
- 01:54
to a transition metal and it'll be putty
- 01:57
in your hands... literally. The doctor
- 01:59
proceeds to heap praise on the metals in
- 02:01
hopes of boosting their self-confidence.
- 02:03
She applauds them for their shiny and
- 02:05
colorful appearances as well as their
- 02:07
high melting and boiling points. Again, all [Transition metals with rainbow above their heads]
- 02:10
stuff that's sure to make a metal feel extra
- 02:12
good about itself. By the time the
- 02:14
transition metals leave their doctor's
- 02:16
office, they're feeling much better about
- 02:18
their place on the periodic table... even
- 02:20
if a few of the alkali earth metals are [Earth metals standing in doctors office]
- 02:22
getting all up in their business.
Related Videos
When you're about to marry the love of your life, not many things could stop you. However, finding out that your future hubby is keeping his crazy...
Here at Shmoop, we work for kids, not just the bottom line. Founded by David Siminoff and his wife Ellen Siminoff, Shmoop was originally conceived...
ACT Math: Elementary Algebra Drill 4, Problem 5. What is the solution to the problem shown?
AP® English Literature and Composition Passage Drill 1, Problem 1. Which literary device is used in lines 31 to 37?
AP® English Literature and Composition Passage Drill 2, Problem 1. What claim does Bacon make that contradicts the maxim "Whatsoever is delig...