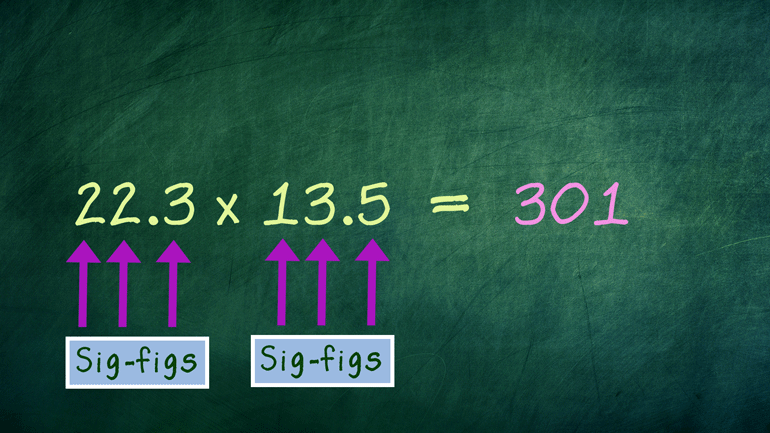ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Chemistry Videos 44 videos
Don't even think about stepping foot into a lab until you drink a nice big cup of safe-ty. Safety lesson number one: do not listen to this descript...
Today we're playing with fire. Wait, we're not supposed to say playing...having fun with fire? Today's lesson is on the colors that can be emitted...
These figures may not be significant to you, but they matter to us, okay? Oh, and to Science. They matter a ton to Science.
Chemistry: 4.8 Photons 46 Views
Share It!
Description:
When you turn on a light, what comes out? Friends...or photons? Well, you'll know the answer as soon as you watch this video.
Transcript
- 00:04
Many great scientific minds have combined their efforts to help us arrive
- 00:08
at our current understanding of what we [Hertz, Einstein and Lenard paintings]
- 00:10
today call the "photoelectric effect,"
- 00:13
which is what happens when light strikes
- 00:15
a metal and the resulting energy
Full Transcript
- 00:18
transfer causes electrons to be emitted.
- 00:21
Of course, not all of these scientific [Hertz stood in a lab]
- 00:23
greats performed their experiments in the
- 00:24
same place and at the same time but it's
- 00:26
a whole lot more fun to pretend that they did, so
- 00:29
here we go. First, we've got the German
- 00:31
physicist Heinrich Hertz, who discovered [Hertz waving in a lab]
- 00:34
while working with a spark-gap
- 00:36
transmitter, which is an über-old radio
- 00:39
broadcasting device, that substances
- 00:41
would sometimes give off a spark after
- 00:43
absorbing light. Rather than chalking the
- 00:46
phenomenon up to it being late and the
- 00:48
fact that he hadn't slept much in the
- 00:49
past week, Hertz continued to study the [Hertz using a flash light on the spark-gap transmitter]
- 00:52
effect, which has been sometimes
- 00:53
referred to as the "Hertz effect." So yeah,
- 00:57
nothing to do with rental cars. The next [Hertz appears at a car rental store]
- 00:59
guy to throw his hat into the lab was
- 01:01
Phillipp Lenard, who was a former
- 01:04
assistant of Hertz's. So it makes sense that [Lenard walks into the lab]
- 01:07
they'd both be there, right? Well, Lenard
- 01:09
piggybacked on Hertz's research and
- 01:11
eventually put together a little [Lenard giving Hertz a piggyback]
- 01:12
experiment of his own that shed a lot of...
- 01:14
light... on the subject. He wanted to test
- 01:17
the theory of thermal emission posed by
- 01:20
classical mechanics that when light
- 01:23
shines on a surface, the transfer of
- 01:25
energy to that surface increases the
- 01:28
energy of the particles, which in turn
- 01:30
emit electrons. So, he built this thing:
- 01:33
cathode here, anode here, and this thing [Finger points to cathode and anode]
- 01:36
is a vacuum tube, and an ammeter here to
- 01:39
measure the current. Well, when Lenard let
- 01:42
lights of varying intensity reach the [Lenard using a flash light on the transmitter]
- 01:44
cathode, he found that below a certain
- 01:46
threshold frequency, no electrons at all
- 01:49
were emitted, while above that threshold
- 01:51
frequency, the number of electrons that
- 01:54
made their way to the anode was
- 01:55
proportional to the intensity of the
- 01:58
light. So what Lenard observed flew a bit
- 02:00
in the face of classical physics. There [Electrons flying around Lenard]
- 02:02
had to be some mysterious explanation
- 02:05
for what was happening. Enter Albert
- 02:07
Einstein. Go ahead, squeeze on in
- 02:09
there Albert; they'll make space for you. [Einstein squeezes in between Hertz and Lenard]
- 02:11
Albert introduced the idea of "photons,"
- 02:14
basically packets of light that aren't
- 02:16
just waves, but also actual particles of
- 02:19
energy. Einstein's theory of
- 02:21
wave-particle duality proposed that [Electrons moving around]
- 02:23
these photons would strike electrons and
- 02:25
force them to say au revoir to
- 02:27
their atom. Kind of like a new boyfriend
- 02:29
moving in in the old one moving out. You [Boyfriend moves out of house and Lenard appears]
- 02:31
know what we're talking about, there,
- 02:32
Lenard. Anyway, Einstein found that
- 02:35
each electron will only be ejected from
- 02:38
its atom if the frequency of light is [Rainbow colored light with different frequencies]
- 02:41
high enough. Well, this discovery
- 02:43
kickstarted something we now call
- 02:44
"quantum mechanics." Yeah, just those words
- 02:48
are enough to give you a headache, but it
- 02:49
was a huge breakthrough in the
- 02:50
understanding of light, energy, and
- 02:52
thermodynamics, and... Okay, that was bound [Hertz and Lenard shaking hands and Einstein giving thumbs up]
- 02:55
to happen.
Related Videos
When you're about to marry the love of your life, not many things could stop you. However, finding out that your future hubby is keeping his crazy...
Here at Shmoop, we work for kids, not just the bottom line. Founded by David Siminoff and his wife Ellen Siminoff, Shmoop was originally conceived...
ACT Math: Elementary Algebra Drill 4, Problem 5. What is the solution to the problem shown?
AP® English Literature and Composition Passage Drill 1, Problem 1. Which literary device is used in lines 31 to 37?
AP® English Literature and Composition Passage Drill 2, Problem 1. What claim does Bacon make that contradicts the maxim "Whatsoever is delig...